Rhodium-Catalyzed Regioselective C—O and C—C Bonds Formation of 3-Oxopent-4-enenitriles with Alkynes for the Synthesis of Polysubstituted 2H-Pyrans
Corresponding Author
Kelu Yan
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
E-mail: [email protected]Search for more papers by this authorXiao Liu
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
Search for more papers by this authorJiangwei Wen
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
Search for more papers by this authorQiuyun Li
Faculty of Chemistry and Chemical Engineering, Yancheng Institute of Technology, Yancheng, Jiangsu, 224051 China
Search for more papers by this authorJunjie Wang
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
Search for more papers by this authorYang Zheng
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
Search for more papers by this authorXiu Wang
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
Search for more papers by this authorCorresponding Author
Kelu Yan
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
E-mail: [email protected]Search for more papers by this authorXiao Liu
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
Search for more papers by this authorJiangwei Wen
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
Search for more papers by this authorQiuyun Li
Faculty of Chemistry and Chemical Engineering, Yancheng Institute of Technology, Yancheng, Jiangsu, 224051 China
Search for more papers by this authorJunjie Wang
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
Search for more papers by this authorYang Zheng
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
Search for more papers by this authorXiu Wang
Key Laboratory of Life-Organic Analysis of Shandong Province, School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong, 273165 China
Search for more papers by this authorComprehensive Summary
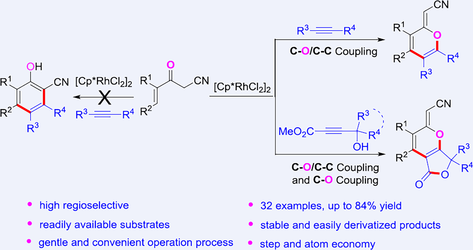
The rhodium-catalyzed C—H bond activation and cyclization of 3-oxopent-4-enenitriles with alkynes proceed efficiently. Various 2H-pyrans with multiple substituents are achieved in good yields through regioselective formation of C—O and C—C bonds. Transformations involving hydroxy-alkynoates resulted in products with a furo[3,4-b]pyran skeleton via further intramolecular ester exchange processes. Different from the traditional “1-oxatrienes pathway”, this method for the synthesis of useful 2H-pyrans possesses certain highlights in terms of readily available substrates, stable and easily derivatized products, gentle and convenient operation process, and step and atom economy.
Supporting Information
| Filename | Description |
|---|---|
| cjoc_202400239_sm_suppl.pdfPDF document, 4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Hsung, R. P.; Kurdyumov, A. V.; Sydorenko, N. A Formal [3 + 3] Cycloaddition Approach to Natural-Product Synthesis. Eur. J. Org. Chem. 2005, 23–44; (b) Tang, Y.; Oppenheimer, J.; Song, Z.; You, L.; Zhang, X.; Hsung, R. P. Strategies and Approaches for Constructing 1-Oxadecalins. Tetrahedron 2006, 62, 10785–10813; (c) Xie, P.; Yang, J.; Zheng, J.; Huang, Y. Sequential Catalyst Phosphine/Secondary Amine Promoted [1+4]/Rearrangement Domino Reaction for the Construction of (2H)-Pyrans and 2-Oxabicyclo[2.2.2]oct-5-ene Skeletons. Eur. J. Org. Chem. 2014, 1189–1194.
- 2(a) Malerich, J. P.; Maimone, T. J.; Elliott, G. I.; Trauner, D. Biomimetic Synthesis of Antimalarial Naphthoquinones. J. Am. Chem. Soc. 2005, 127, 6276–6283; (b) Beaudry, C. M.; Malerich, J. P.; Trauner, D. Biosynthetic and Biomimetic Electrocyclizations. Chem. Rev. 2005, 105, 4757–4778; (c) Riveira, M. J.; La-Venia, A.; Mischne, M. P. Pericyclic Cascade toward Isochromenes: Application to the Synthesis of Alkaloid Benzosimuline. J. Org. Chem. 2016, 81, 7977–7983; (d) Li, K.; Ou, J.; Gao, S. Total Synthesis of Camptothecin and Related Natural Products by a Flexible Strategy. Angew. Chem. Int. Ed. 2016, 55, 14778–14783; (e) Suzuki, T.; Watanabe, S.; Kobayashi, S.; Tanino, K. Enantioselective Total Synthesis of (+)-Iso-A82775C, a Proposed Biosynthetic Precursor of Chloropupukeananin. Org. Lett. 2017, 19, 922–925; (f) Hall, A. J.; Roche, S. P.; West, L. M. Synthesis of Briarane Diterpenoids: Biomimetic Transannular Oxa-6π electrocyclization Induced by a UVA/UVC Photoswitch. Org. Lett. 2017, 19, 576–579.
- 3(a) Peng, W.; Hirabaru, T.; Kawafuchi, H.; Inokuchi, T. Substituent-Controlled Electrocyclization of 2,4-Dienones: Synthesis of 2,3,6-Trisubstituted 2H-Pyran-5-carboxylates and Their Transformations. Eur. J. Org. Chem. 2011, 5469–5474; (b) Fotiadou, A. D.; Zografos, A. L. Electrocyclization of Oxatrienes in the Construction of Structurally Complex Pyranopyridones. Org. Lett. 2012, 14, 5664–5667; (c) Riveira, M. J.; Mischne, M. P. Green One-Pot Synthesis of 2H-Pyrans Under Solvent-Free Conditions Catalyzed by Ethylenediammonium Diacetate. Synth. Commun. 2013, 43, 208–220; (d) Jacob, S. D.; Brooks, J. L.; Frontier, A. J. No Acid Required: 4π and 6π Electrocyclization Reactions of Dienyl Diketones for the Synthesis of Cyclopentenones and 2H-Pyrans. J. Org. Chem. 2014, 79, 10296–10302.
- 4(a) Zhu, Z. B.; Kirsch, S. F. Propargyl Vinyl Ethers as Heteroatom- Tethered Enyne Surrogates: Diversity-Oriented Strategies for Heterocycle Synthesis. Chem. Commun. 2013, 49, 2272–2283; (b) Tejedor, D.; Méndez-Abt, G.; Cotos, L.; García-Tellado, F. Propargyl Claisen Rearrangement: Allene Synthesis and Beyond. Chem. Soc. Rev. 2013, 42, 458–471; (c) Hashmi, A. S. K.; Graf, K.; Ackermann, M.; Rominger, F. Gold(I)-Catalyzed Domino Reaction of Allyl 2-en-4-ynyl Ethers to 1,3,6-Trien-4-yl Ketones. ChemCatChem 2013, 5, 1200–1204; (d) Vidhani, D. V.; Krafft, M. E.; Alabugin, I. V. Rh(I)-Catalyzed Transformation of Propargyl Vinyl Ethers into (E,Z)-Dienals: Stereoelectronic Role of trans Effect in a Metal-Mediated Pericyclic Process and a Shift from Homogeneous to Heterogeneous Catalysis During a One-Pot Reaction. J. Org. Chem. 2014, 79, 352–364.
- 5(a) Gosink, T. A. Valence Isomers. Substituent Effects on the Equilibrium between 2H-Pyrans and Cis Dienones. J. Org. Chem. 1974, 39, 1942–1944; (b) Rodriguez-Otero, J. Study of the Electrocyclization of (Z)-Hexa-1,3,5-triene and Its Heterosubstituted Analogues Based on Ab Initio and DFT Calculations. J. Org. Chem. 1999, 64, 6842–6848; (c) Krasnaya, Zh. A. Dienone ⇆ 2H-Pyran Valence Isomerization. (Review). Chem. Heterocycl. Compd. 1999, 35, 1255–1271; (d) Riveira, M. J.; Quiroga, G. N.; Mata, E. G.; Gandon, V.; Mischne, M. P. Cycloisomerization of Conjugated Trienones and Isomeric 2H-Pyrans: Unified Strategy toward Cyclopenta[b]furans. J. Org. Chem. 2015, 80, 6515–6519.
- 6(a) Rauser, M.; Schroeder, S.; Niggemann, M. Cooperative Catalysis: Calcium and Camphorsulfonic Acid Catalyzed Cycloisomerization of Diynols. Chem. Eur. J. 2015, 21, 15929–15933; (b) Tejedor, D.; Díaz-Díaz, A.; Diana-Rivero, R.; Delgado-Hernández, S.; García-Tellado, F. Synthesis and Utility of 2,2-Dimethyl-2H-pyrans: Dienes for Sequential Diels−Alder/Retro-Diels−Alder Reactions. Org. Lett. 2018, 20, 7987−7990.
- 7(a) Yang, Y.; Lan, J.; You, J. Oxidative C–H/C–H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863; (b) Gandeepan, P.; Muller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452; (c) Rej, S.; Ano, Y.; Chatani, N. Bidentate Directing Groups: An Efficient Tool in C–H Bond Functionalization Chemistry for the Expedient Construction of C–C Bonds. Chem. Rev. 2020, 120, 1788–1887; (d) Mandal, D.; Roychowdhury, S.; Biswas, J. P.; Maiti, S.; Maiti, D. Transition-Metal-Catalyzed C–H Bond Alkylation Using Olefins: Recent Advances and Mechanistic Aspects. Chem. Soc. Rev. 2022, 51, 7358–7426; (e) He, Y.; Huang, Z.; Wu, K.; Ma, J.; Zhou, Y.-G.; Yu, Z. Recent Advances in Transition-Metal-Catalyzed Carbene Insertion to C–H Bonds. Chem. Soc. Rev. 2022, 51, 2759–2852.
- 8(a) Thirunavukkarasu, V. S.; Donati, M.; Ackermann, L. Hydroxyl-Directed Ruthenium-Catalyzed C–H Bond Functionalization: Versatile Access to Fluorescent Pyrans. Org. Lett. 2012, 14, 3416−3419; (b) Liao, G.; Song, H.; Yin, X.-S.; Shi, B.-F. Expeditious Synthesis of Pyrano[2,3,4-de]-quinolines via Rh(III)-Catalyzed Cascade C–H Activation/Annulation/Lactonization of Quinolin-4-ol with Alkynes. Chem. Commun. 2017, 53, 7824–7827; (c) Mei, R.; Koeller, J.; Ackermann, L. Electrochemical Ruthenium-Catalyzed Alkyne Annulations by C–H/Het–H Activation of Aryl Carbamates or Phenols in Protic Media. Chem. Commun. 2018, 54, 12879–12882; (d) Yan, K.; Li, B.; Wang, B. Hydroxyl-Directed Rhodium-Catalyzed C–H Bond Activation and Cyclization Leading to Naphtho[1,8-bc]pyran Derivatives and Its Analogues. Adv. Synth. Catal. 2018, 360, 2113–2118; (e) Dutta, P. K.; Ravva, M. K.; Sen, S. Cobalt-Catalyzed, Hydroxyl-Assisted C−H Bond Functionalization: Access to Diversely Substituted Polycyclic Pyrans. J. Org. Chem. 2019, 84, 1176−1184; (f) Ma, W.; Tan, Y.; Wang, Y.; Li, Z.; Li, Z.; Gu, L.; Mei, R.; Cheng, A. Hydroxyl-Directed Ruthenium-Catalyzed peri-Selective C−H Acylmethylation and Annulation of Naphthols with Sulfoxonium Ylides. Org. Lett. 2021, 23, 6200−6205; (g) Li, L.; Zhong, X.; Xu, J.; Gao, H.; Zhou, Z.; Yi, W. Synthesis of Difluorinated Dihydrobenzo[de]chromenes via Rh(III)-Catalysed C-H Couplings of 1-Naphthols with Gem-Difluoromethylene Alkynes. Adv. Synth. Catal. 2021, 363, 1352–1357; (h) Yang, Z.; Tang, J.; Chen, Z.; Wu, X.-F. Ruthenium-Catalyzed Hydroxyl-Directed peri-Selective C−H Activation and Annulation of 1-Naphthols with CF3–Imidoyl Sulfoxonium Ylides for the Synthesis of 2–(Trifluoromethyl)-2,3-dihydrobenzo[de]chromen-2-amines. Org. Lett. 2022, 24, 7288−7293; (i) Ren, J.; Pi, C.; Cui, X.; Wu, Y. Transition Metal-Controlled Divergent Annulations of Azomethine Imines with Iodonium Ylides via C-Centered [1,2]-Rearrangement. Org. Lett. 2023, 25, 2582−2587; (j) Lv, G.; Zhang, Q.; Zhang, C.; Chen, Y.; Lin, Z.; Lai, R.; Yang, Z.; Wu, Y. The Pyridotriazole Works as a Traceless Directing Group: A C−H Activation/Annulation Cascade Reaction with Iodonium Ylides. Org. Lett. 2023, 25, 4022−4027.
- 9(a) Mochida, S.; Hirano, K.; Satoh, T.; Miura, M. Synthesis of Functionalized α-Pyrone and Butenolide Derivatives by Rhodium-Catalyzed Oxidative Coupling of Substituted Acrylic Acids with Alkynes and Alkenes. J. Org. Chem. 2009, 74, 6295–6298; (b) Itoh, M.; Shimizu, M.; Hirano, K.; Satoh, T.; Miura, M. Rhodium-Catalyzed Decarboxylative and Dehydrogenative Coupling of Maleic Acids with Alkynes and Alkenes. J. Org. Chem. 2013, 78, 11427−11432; (c) Wu, J.; Wang, D.; Wan, Y.; Ma, C. Rhodium-Catalyzed Tunable Oxidative Cyclization toward the Selective Synthesis of α-Pyrones and Furans. Chem. Commun. 2016, 52, 1661–1664; (d) Zhou, P.; Yang, W.-T.; Rahman, A. U.; Li, G.; Jiang, B. Rh(III)-Catalyzed [3 + 3] Annulation Reaction of Cyclopropenones and Sulfoxonium Ylides toward Trisubstituted 2-Pyrones. J. Org. Chem. 2020, 85, 360−366; (e) Hong, C.; Yu, S.; Liu, Z.; Zhang, Y. Rh-Catalyzed Coupling of Acrylic/Benzoic Acids with α-Diazocarbonyl Compounds: An Alternative Route for α-Pyrones and Isocoumarins. Org. Lett. 2022, 24, 815−820.
- 10Fu, L.; Xu, W.; Pu, M.; Wu, Y.-D.; Liu, Y.; Wan, J.-P. Rh-Catalyzed [4 + 2] Annulation with a Removable Monodentate Structure toward Iminopyranes and Pyranones by C−H Annulation. Org. Lett. 2022, 24, 3003−3008.
- 11Ferguson, J.; Zeng, F.; Alper, H. Synthesis of Coumarins via Pd-Catalyzed Oxidative Cyclocarbonylation of 2-Vinylphenols. Org. Lett. 2012, 14, 5602−5605.
- 12(a) Li, X. G.; Sun, M.; Liu, K.; Jin, Q.; Liu, P. N. Rh(III)-Catalyzed C–H Activation/Cyclization of Benzamides and Diazo Compounds to Form Isocoumarins and α-Pyrones. Chem. Commun. 2015, 51, 2380–2383; (b) Huang, Y.; Lyu, X.; Song, H.; Wang, Q. Sulfoxonium Ylides as Carbene Precursors: Rhodium(III)-Catalyzed Sequential C–H Functionalization, Selective Enol Oxygen-Atom Nucleophilic Addition, and Hydrolysis. Adv. Synth. Catal. 2019, 361, 5272–5276; (c) Dong, G.; Li, C.; Liu, H. Rh(III)-Catalyzed Annulation of Boc-Protected Benzamides with Diazo Compounds: Approach to Isocoumarin. Molecules 2019, 24, 937; (d) Yin, C.; Li, L.; Yu, C. Rh(III)-Catalyzed C–H Annulation of Sulfoxonium Ylides with Iodonium Ylides towards Isocoumarins. Org. Biomol. Chem. 2022, 20, 1112–1116; (e) Wang, Q.; Li, Y.; Sun, J.; Chen, S.; Li, H.; Zhou, Y.; Li, J.; Liu, H. Rh-Catalyzed C−H Activation/Annulation of Enaminones and Cyclic 1,3-Dicarbonyl Compounds: An Access to Isocoumarins. J. Org. Chem. 2023, 88, 5348–5358.
- 13(a) Baruah, S.; Kaishap, P. P.; Gogoi, S. Ru(II)-Catalyzed C–H Activation and Annulations of Salicylaldehydes with Monosubstituted and Disubstituted Alkynes. Chem. Commun. 2016, 52, 13004–13007; (b) Sun, P.; Gao, S.; Yang, C.; Guo, S.; Lin, A.; Yao, H. Controllable Rh(III)-Catalyzed Annulation between Salicylaldehydes and Diazo Compounds: Divergent Synthesis of Chromones and Benzofurans. Org. Lett. 2016, 18, 6464−6467; (c) Raja, G. C. E.; Ryu, J. Y.; Lee, J.; Lee, S. Ruthenium-Catalyzed C−H Activation of Salicylaldehyde and Decarboxylative Coupling of Alkynoic Acids for the Selective Synthesis of Homoisoflavonoids and Flavones. Org. Lett. 2017, 19, 6606–6609; (d) Cai, L.; Zhu, X.; Chen, J.; Lin, A.; Yao, H. Rh(III)-Catalyzed C–H Activation/Annulation of Salicylaldehydes with Sulfoxonium Ylides for the Synthesis of Chromones. Org. Chem. Front. 2019, 6, 3688–3692.
- 14Tan, X.; Liu, B.; Li, X.; Li, B.; Xu, S.; Song, H.; Wang, B. Rhodium-Catalyzed Cascade Oxidative Annulation Leading to Substituted Naphtho[1,8-bc]pyrans by Sequential Cleavage of C(sp2)–H/C(sp3)–H and C(sp2)–H/O–H Bonds. J. Am. Chem. Soc. 2012, 134, 16163–16166.
- 15(a) Zhou, T.; Li, B.; Wang, B. Rhodium-Catalyzed C–H Activation of 3-(Indolin-1-yl)-3-oxopropanenitriles with Diazo Compounds and Tandem Cyclization Leading to Hydrogenated Azepino[3,2,1-hi]indoles. Chem. Commun. 2016, 52, 14117–14120; (b) Zhou, T.; Wang, Y.; Li, B.; Wang, B. Rh(III)-Catalyzed Carbocyclization of 3-(Indolin-1- yl)-3-oxopropanenitriles with Alkynes and Alkenes through C–H Activation. Org. Lett. 2016, 18, 5066–5069; (c) Zhou, T.; Li, B.; Wang, B. Rhodium-Catalyzed C2 and C4 C–H Activation/Annulation of 3-(1H-Indol-3-yl)-3-oxopropanenitriles with Internal Alkynes: A Facile Access to Substituted and Fused Carbazoles. Chem. Commun. 2017, 53, 6343–6346; (d) Yan, K.; Li, B.; Wang, B. Iridium-Catalyzed Tandem Cyclization of Benzoylacetonitriles with Diazo Compounds Leading to Substituted Naphtho[1,8-bc]pyrans by Sequential C−H Functionalization. Adv. Synth. Catal. 2018, 360, 2272–2279; (e) Li, Q.; Li, B.; Wang, B. Rhodium-Catalyzed Intramolecular Cascade Sequence for the Formation of Fused Carbazole-Annulated Medium-Sized Rings by Cleavage of C(sp2)–H/C(sp3)–H Bonds. Chem. Commun. 2018, 54, 9147–9150; (f) Xiao, Y.; Xiong, H.; Sun, S.; Yu, J.; Cheng, J. Rh(iii)-Catalyzed Dual C–H Functionalization of 3-(1H-Indol-3-yl)-3- oxopropanenitriles with Sulfoxonium Ylides or Diazo Compounds toward Polysubstituted Carbazoles. Org. Biomol. Chem. 2018, 16, 8715–8718; (g) Fang, F.; Zhang, C.; Zhou, C.; Li, Y.; Zhou, Y.; Liu, H. Rh(III)-Catalyzed C–H Activation of Benzoylacetonitriles and Tandem Cyclization with Diazo Compounds to Substituted Benzo[de]chromenes. Org. Lett. 2018, 20, 1720−1724; (h) Song, X.; Doan, B. N. D.; Zhang, X.; Lee, R.; Fan, X. Complementary C–H Functionalization Mode of Benzoylacetonitriles: Computer-Augmented Study of a Regio- and Stereoselective Synthesis of Functionalized Benzofulvenes. Org. Lett. 2020, 22, 46–51; (i) Kotipalli, R.; Babu, U. S.; Nanubolu, J. B.; Reddy, M. S. Rh-Catalyzed Chemo-, Stereo- and Regioselective C–H Cascade Annulation of Indolyloxopropanenitriles for Pyranoindoles. Chem. Commun. 2023, 59, 10137–10140.
- 16Liu, M.; Yan, K.; Wen, J.; Li, X.; Wang, X.; Lu, F.; Wang, X.; Wang, H. Synthesis of Polysubstituted Phenols by Rhodium-Catalyzed C–H/Diazo Coupling and Tandem Annulation. Adv. Synth. Catal. 2021, 363, 1855–1860.
- 17(a) Yan, K.; Liu, M.; Wen, J.; Wang, S.; Li, J.; Wang, H. Synthesis of Substituted Naphtho[1,8-bc]thiopyrans by Sulfhydryl-Directed Rhodium-Catalyzed peri-Selective C–H Bond Activation and Cyclization of Naphthalene-1-thiols. Org. Lett. 2020, 22, 7825–7830; (b) Yan, K.; Liu, M.; Wen, J.; Liu, X.; Wang, X.; Chen, X.; Li, J.; Wang, S.; Wang, X.; Wang, H. Visible-Light-Promoted Cascade Cyclization towards Benzo[d]imidazo[5,1-b]thiazoles under Metal- and Photocatalyst- Free Conditions. Green Chem. 2021, 23, 1286–1291; (c) Yan, K.; Liu, M.; Wen, J.; Liu, W.; Li, X.; Liu, X.; Sui, X.; Shang, W.; Wang, X. Copper-Catalyzed Domino Synthesis of Benzo[d]imidazo [5,1-b][1,3]selenazoles Involving Sequential Intermolecular Cycloaddition and Intramolecular Ullmann-Type C–Se Bond Formation. Org. Chem. Front. 2021, 8, 5139–5144; (d) Liu, M.; Yan, K.; Wen, J.; Liu, W.; Wang, M.; Wang, L.; Wang, X. Synthesis of Substituted 1-Hydroxy-2-Naphthaldehydes by Rhodium-Catalyzed C–H Bond Activation and Vinylene Transfer of Enaminones with Vinylene Carbonate. Adv. Synth. Catal. 2022, 364, 512–517; (e) Liu, M.; Yan, K.; Wen, J.; Shang, W.; Sui, X.; Wang, X. Ruthenium-Catalyzed C7-Formylmethylation or Sequential Acetalization of Indolines with Vinylene Carbonate in Different Solvents. Adv. Synth. Catal. 2022, 364, 1580–1586.
- 18(a) Wang, K.; Hu, F.; Zhang, Y.; Wang, J. Directing Group-Assisted Transition-Metal-Catalyzed Vinylic C–H Bond Functionalization. Sci. China Chem. 2015, 58, 1252–1265;
(b) Besset, T.; Kuhl, N.; Patureau, F. W.; Glorius, F. RhIII-Catalyzed Oxidative Olefination of Vinylic C–H Bonds: Efficient and Selective Access to Di-unsaturated α-Amino Acid Derivatives and Other Linear 1,3-Butadienes. Chem. Eur. J. 2011, 17, 7167–7170;
(c) Liang, Q.-J.; Yang, C.; Meng, F.-F.; Jiang, B.; Xu, Y.-H.; Loh, T.-P. Chelation versus Non-Chelation Control in the Stereoselective Alkenyl sp2 C−H Bond Functionalization Reaction. Angew. Chem. Int. Ed. 2017, 56, 5091–5095;
(d) Jiang, B.; Zhao, M.; Li, S.-S.; Xu, Y.-H.; Loh, T.-P. Macrolide Synthesis through Intramolecular Oxidative Cross-Coupling of Alkenes. Angew. Chem. Int. Ed. 2018, 57, 555–559;
(e) Zhang, J.; Lu, X.; Shen, C.; Xu, L.; Ding, L.; Zhong, G. Recent Advances in Chelation-Assisted Site- and Stereoselective Alkenyl C–H Functionalization. Chem. Soc. Rev. 2021, 50, 3263–3314;
(f) Zhu, Y.; Wang, Y.; Shen, W.; Chen, X.; Liu, Q.; Yang, L.; Zhong, G.; Zhang, J. Stereoselective Synthesis of Complex Polyenes through Sequential α-/β-C−H Functionalization of trans-Styrenes. Angew. Chem. Int. Ed. 2024, 136, e202315273.
10.1002/ange.202315273 Google Scholar
- 19Giussi, J. M.; Ponzinibbio, A.; Cortizo, M. S.; Allegretti, P. E. 3-Hydroxy-4-methyl-4-pentenonitrile and 4-Methyl-3-oxo-4-pentenonitrile: Study of the Tautomerics Equilibria in Gas Phase and in Solution. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 2010, 77, 367–373.




