Copper/Photoredox-Catalyzed Regioselective 1,4-Addition to 1,3-Enynes: A Convenient Access to Perfluoroalkylated Allenes
Corresponding Author
Ya Chen
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
E-mail: [email protected]; [email protected]Search for more papers by this authorQuanyuan Wanga
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
Search for more papers by this authorKeyi Penga
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
Search for more papers by this authorLilei Chenga
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
Search for more papers by this authorZiyi Shea
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
Search for more papers by this authorCorresponding Author
Guo-Jun Deng
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
Department School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ya Chen
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
E-mail: [email protected]; [email protected]Search for more papers by this authorQuanyuan Wanga
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
Search for more papers by this authorKeyi Penga
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
Search for more papers by this authorLilei Chenga
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
Search for more papers by this authorZiyi Shea
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
Search for more papers by this authorCorresponding Author
Guo-Jun Deng
Key Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan, Hunan, 411105 China
Department School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
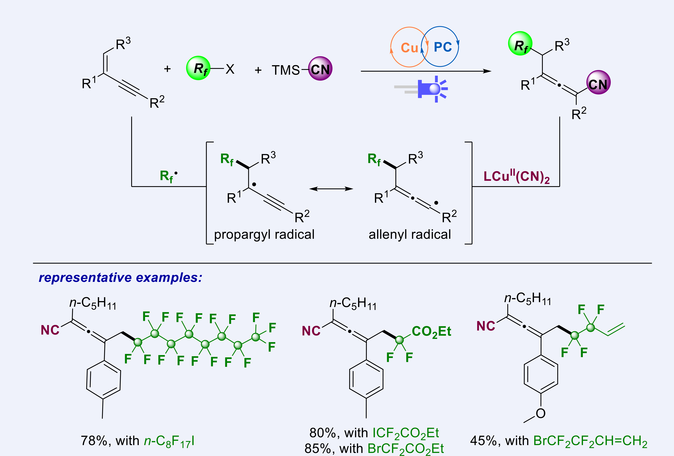
A general and broadly applicable copper and photoredox dual-catalyzed multicomponent 1,4-perfluoroalkylcyanation of 1,3-enynes has been developed. This protocol enjoys success with high regioselectivity, mild reaction conditions, and excellent functional-group tolerance, allowing the facile synthesis of structurally diverse perfluoroalkylated allenes from readily available fluoroalkyl halides, 1,3-enynes and TMSCN in a one-pot manner. A reasonable mechanism has been proposed according to a series of control experiments.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400207-sup-0001-supinfo.pdfPDF document, 7.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed., Wiley-VCH, Weinheim, Germany, 2013;
10.1002/9783527651351 Google Scholar(b) Müller, K.; Faeh, C.; Diederich, F. o. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886.
- 2(a) Chu, L.; Qing, F.-L. Oxidative Trifluoromethylation and Trifluoromethylthiolation Reactions Using (Trifluoromethyl)trimethylsilane as a Nucleophilic CF3 Source. Acc. Chem. Res. 2014, 47, 1513–1522; (b) Ni, C.; Hu, M.; Hu, J. Good Partnership between Sulfur and Fluorine: Sulfur-Based Fluorination and Fluoroalkylation Reagents for Organic Synthesis. Chem. Rev. 2015, 115, 765–825; (c) Xie, Q.; Hu, J. Chen's Reagent: A Versatile Reagent for Trifluoromethylation, Difluoromethylenation, and Difluoroalkylation in Organic Synthesis. Chin. J. Chem. 2020, 38, 202–212.
- 3(a) Li, Z.; García-Domínguez, A.; Nevado, C. Pd-Catalyzed Stereoselective Carboperfluoroalkylation of Alkynes. J. Am. Chem. Soc. 2015, 137, 11610–11613; (b) Domański, S.; Chaładaj, W. A Broadly Applicable Method for Pd-Catalyzed Carboperfluoro-alkylation of Terminal and Internal Alkynes: A Convenient Route to Tri- and Tetrasubstituted Olefins. ACS Catal. 2016, 6, 3452–3456; (c) Kawamura, S.; Sodeoka, M. Perfluoroalkylation of Unactivated Alkenes with Acid Anhydrides as the Perfluoroalkyl Source. Angew. Chem. Int. Ed. 2016, 55, 8740–8743; (d) Lin, J.-S.; Wang, F.-L.; Dong, X.-Y.; He, W.-W.; Yuan, Y.; Chen, S.; Liu, X.-Y. Catalytic Asymmetric Radical Aminoperfluoroalkylation and Aminodifluoromethylation of Alkenes to Versatile Enantioenriched-Fluoroalkyl Amines. Nat. Commun. 2017, 8, 14841; (e) Xiong, H.; Ramkumar, N.; Chiou, M.-F.; Jian, W.; Li, Y.; Su, J.-H.; Zhang, X.; Bao, H. Iron-Catalyzed Carboazidation of Alkenes and Alkynes. Nat. Commun. 2019, 10, 122.
- 4(a) Yamazaki, T.; Yamamoto, T.; Ichihara, R. Preparation of CF3-Containing 1,3-Di- and 1,1,3-Trisubstituted Allenes. J. Org. Chem. 2006, 71, 6251–6253; (b) Han, H. Y.; Kim, M. S.; Son, J. B.; Jeong, I. H. Novel Synthesis of 1-Aryl-1-Trifluoromethylallenes. Tetrahedron Lett. 2006, 47, 209–212.
- 5(a) Watanabe, Y.; Yamazaki, T. Divergent Preparation of Allenyl Tosylates and α-Tosyloxy Ketones by Facile and Efficient Isomerization of CF3-Containing Propargylic Tosylates. J. Fluorine Chem. 2010, 131, 646–651; (b) Blanchard, N.; Evano, G.; Guissart, C.; Dolbois, A.; Tresse, C.; Saint-Auret, S. A Straightforward Entry to γ-Trifluoromethylated Allenamides and Their Synthetic Applications. Synlett 2016, 27, 2575–2580.
- 6 Boreux, A.; Lonca, G. H.; Riant, O.; Gagosz, F. Synthesis of Trifluoromethyl-allenes by Gold-Catalyzed Rearrangement of Propargyl Benzyl Ethers. Org. Lett. 2016, 18, 5162–5165.
- 7(a) Yamazaki, T.; Watanabe, Y. Facile Preparation of CF3-Containing 1-Bromoallenes. Synlett 2009, 20, 3352–3354;
10.1055/s-0029-1218383 Google Scholar(b) Aikawa, K.; Hioki, Y. t.; Mikami, K. Highly Enantioselective Alkynylation of Trifluoropyruvate with Alkynylsilanes Catalyzed by the BINAP−Pd Complex: Access to α-Trifluoromethyl-Substituted Tertiary Alcohols. Org. Lett. 2010, 12, 5716–5719.
- 8(a) Zhao, T. S. N.; Szabó, K. J. Trifluoromethylation of Propargylic Halides and Trifluoroacetates Using (Ph3P)3Cu(CF3) Reagent. Org. Lett. 2012, 14, 3966–3969; (b) Miyake, Y.; Ota, S.; Shibata, M.; Nakajima, K.; Nishibayashi, Y. Copper-Catalyzed Nucleophilic Trifluoromethylation of Propargylic Halides. Chem. Commun. 2013, 49, 7809–7811; (c) Ambler, B. R.; Peddi, S.; Altman, R. A. Ligand-Controlled Regioselective Copper-Catalyzed Trifluoromethylation to Generate (Trifluoromethyl)allenes. Org. Lett. 2015, 17, 2506–2509.
- 9 Song, T.; Zhu, L.; Li, H.; Tung, C.-H.; Lan, Y.; Xu, Z. Kinetically Controlled Radical Addition/Elimination Cascade: From Alkynyl Aziridine to Fluorinated Allenes. Org. Lett. 2020, 22, 2419–2424.
- 10(a) Coppola, G. A.; Pillitteri, S.; Van der Eycken, E. V.; You, S.-L.; Sharma, U. K. Multicomponent Reactions and Photo/Electrochemistry Join Forces: Atom Economy Meets Energy Efficiency. Chem. Soc. Rev. 2022, 51, 2313–2382;
(b) Guo, X.; Hu, W. Novel Multicomponent Reactions via Trapping of Protic Onium Ylides with Electrophiles. Acc. Chem. Res. 2013, 46, 2427–2440;
(c) Wang, D.; Yang, Y.; Xiao, F.; Liu, J.; Mao, G.; Deng, G.-J. Synthesis of Biheteroaryls via 2-Methyl Quinoline C(sp3)-H Functionalization under Metal-free Conditions. Green Synth. Catal. 2024, 5, 73–76;
(d) Chen, J.; Jiang, P.; Liu, X.; Huang, H.; Mao, G.; Deng, G.-J. Pictet-Spengler/Transamination Cascade Reaction of Indoles for Modular Synthesis of Marinoquinoline Analogues. Green Synth. Catal. 2023, DOI: https://doi.org/10.1016/j.gresc.2023.09.002.
10.1016/j.gresc.2023.09.002 Google Scholar
- 11(a) Dherbassy, Q.; Manna, S.; Talbot, F. J. T.; Prasitwatcharakorn, W.; Perry, G. J. P.; Procter, D. J. Copper-catalyzed functionalization of enynes. Chem. Sci. 2020, 11, 11380–11393; (b) Fu, L.; Greßies, S.; Chen, P.; Liu, G. Recent Advances and Perspectives in Transition Metal-Catalyzed 1,4-Functionalizations of Unactivated 1,3-Enynes for the Synthesis of Allenes. Chin. J. Chem. 2020, 38, 91–100.
- 12 Li, Y.; Bao, H. Radical Transformations for Allene Synthesis. Chem. Sci. 2022, 13, 8491–8506.
- 13(a) Song, Y.; Fu, C.; Ma, S. Copper-Catalyzed Syntheses of Multiple Functionalizatized Allenes via Three-Component Reaction of Enynes. ACS Catal. 2021, 11, 10007–10013; (b) Miao, H.-J.; Zhang, J.-H.; Liu, S.; Wang, W.-H.; Yang, X.; Duan, X.-H.; Guo, L.-N. Alkoxyl Radical-Mediated Ring Expansion/1,4-Difunctionalization of 1,3-Enynes upon Copper Catalysis. Org. Lett. 2023, 25, 5563–5568.
- 14(a) Xu, T.; Wu, S.; Zhang, Q.-N.; Wu, Y.; Hu, M.; Li, J.-H. Dual Photoredox/Nickel-Catalyzed 1,4-Sulfonylarylation of 1,3-Enynes with Sulfinate Salts and Aryl Halides: Entry into Tetrasubstituted Allenes. Org. Lett. 2021, 23, 8455–8459; (b) Hu, D.-D.; Gao, Q.; Dai, J.-C.; Cui, R.; Li, Y.-B.; Li, Y.-M.; Zhou, X.-G.; Bian, K.-J.; Wu, B.-B.; Zhang, K.-F.; et al. Visible-Light-Induced, Autopromoted Nickel-Catalyzed Three-Component Arylsulfonation of 1,3-Enynes and Mechanistic Insights. Sci. China. Chem. 2022, 65, 753–761; (c) Lv, Y.; Han, W.; Pu, W.; Xie, J.; Wang, A.; Zhang, M.; Wang, J.; Lai, J. Copper-Catalyzed Regioselective 1,4-Sulfonylcyanation of 1,3-Enynes with Sulfonyl Chlorides and TMSCN. Org. Chem. Front. 2022, 9, 3775–3780.
- 15(a) Ye, C.; Li, Y.; Zhu, X.; Hu, S.; Yuan, D.; Bao, H. Copper-Catalyzed 1,4-Alkylarylation of 1,3-Enynes with Masked Alkyl Electrophiles. Chem. Sci. 2019, 10, 3632–3636;
(b) Zhang, K. F.; Bian, K. J.; Li, C.; Sheng, J.; Li, Y.; Wang, X. S. Nickel-Catalyzed Carbofluoroalkylation of 1,3-Enynes to Access Structurally Diverse Fluoroalkylated Allenes. Angew. Chem. Int. Ed. 2019, 58, 5069–5074;
(c) Sun, Q.; Zhang, X. P.; Duan, X.; Qin, L. Z.; Yuan, X.; Wu, M. Y.; Liu, J.; Zhu, S. S.; Qiu, J. K.; Guo, K. Photoinduced Merging with Copper- or Nickel-Catalyzed 1,4-Cyanoalkylarylation of 1,3-Enynes to Access Multiple Functionalizatized Allenes in Batch and Continuous Flow. Chin. J. Chem. 2022, 40, 1537–1545;
(d) Liu, W.; Liu, C.; Wang, M.; Kong, W. Modular Synthesis of Multifunctionalized CF3-Allenes through Selective Activation of Saturated Hydrocarbons. ACS Catal. 2022, 12, 10207–10221;
(e) Cheng, C.; Lv, G.-F.; Wu, S.; Li, Y.; Li, J.-H. Nickel Photocatalyzed Reductive 1,4-Dicarbofunctionalization of 1,3-Enynes with N-Methylamines and Organohalides Enabled by Site-Selective C(sp3)–H Functionalization. Org. Lett. 2023, 25, 4236–4240;
(f) Zhu, C.; Chen, H.; Yue, H.; Rueping, M. Electrochemical Chemo- and Regioselective Arylalkylation, Dialkylation and Hydro(deutero)alkylation of 1,3-Enynes. Nat. Syn. 2023, 2, 1068–1081.
10.1038/s44160-023-00349-9 Google Scholar
- 16(a) Huang, J.; Jia, Y.; Li, X.; Duan, J.; Jiang, Z.-X.; Yang, Z. Halotrifluoromethylation of 1,3-Enynes: Access to Tetrasubstituted Allenes. Org. Lett. 2021, 23, 2314–2319; (b) Li, X.; Li, N.; Yang, L.; Jiang, R.; He, Y.; Shi, J.; Jiang, Z.-X.; Yang, Z. Switchable Nucleophilic Site Enables Expedient Synthesis of CF3-Containing Thiazoles and Allenes from 1,3-Enynes. ACS Catal. 2023, 13, 12755–12765; (c) Li, S.; Yang, W.; Shi, J.; Dan, T.; Han, Y.; Cao, Z.-C.; Yang, M. Synthesis of Trifluoromethyl-Substituted Allenols via Catalytic Trifluoromethylbenzoxylation of 1,3-Enynes. ACS Catal. 2023, 13, 2142–2148.
- 17(a) Dong, X. Y.; Zhan, T. Y.; Jiang, S. P.; Liu, X. D.; Ye, L.; Li, Z. L.; Gu, Q. S.; Liu, X. Y. Copper-Catalyzed Asymmetric Coupling of Allenyl Radicals with Terminal Alkynes to Access Tetrasubstituted Allenes. Angew. Chem. Int. Ed. 2021, 60, 2160–2164; (b) He, F.-S.; Bao, P.; Yu, F.; Zeng, L.-H.; Deng, W.-P.; Wu, J. Copper-Catalyzed Regioselective 1,4-Selenosulfonylation of 1,3-Enynes to Access Cyanoalkylsulfonylated Allenes. Org. Lett. 2021, 23, 7472–7476; (c) Zhang, F.-H.; Guo, X.; Zeng, X.; Wang, Z. Asymmetric 1,4-Functionalization of 1,3-Enynes via Dual Photoredox and Chromium Catalysis. Nat. Commun. 2022, 13, 5036; (d) Chen, H.; Zhu, C.; Yue, H.; Rueping, M. Group 14 Elements Hetero-Difunctionalizations via Nickel-Catalyzed Electroreductive Cross-Coupling. Angew. Chem. Int. Ed. 2023, 62, e202306498; (e) Song, H.; Zhang, X.; Chen, G.; He, X.; Lian, Z. Copper-Catalyzed 1,4-Trifluoromethylthio-Arylsulfonylation of 1,3-Enynes via the Insertion of Sulfur Dioxide. Org. Lett. 2023, 25, 5916–5921.
- 18 Wang, F.; Wang, D.; Zhou, Y.; Liang, L.; Lu, R.; Chen, P.; Lin, Z.; Liu, G. Divergent Synthesis of CF3-Substituted Allenyl Nitriles by Ligand-Controlled Radical 1,2- and 1,4-Addition to 1,3-Enynes. Angew. Chem. Int. Ed. 2018, 57, 7140–7145.
- 19(a) Zhu, X.; Deng, W.; Chiou, M.-F.; Ye, C.; Jian, W.; Zeng, Y.; Jiao, Y.; Ge, L.; Li, Y.; Zhang, X.; Bao, H. Copper-Catalyzed Radical 1,4-Difunctionalization of 1,3-Enynes with Alkyl Diacyl Peroxides and N-Fluorobenzenesulfonimide. J. Am. Chem. Soc. 2019, 141, 548–559; (b) Zeng, Y.; Chiou, M.-F.; Zhu, X.; Cao, J.; Lv, D.; Jian, W.; Li, Y.; Zhang, X.; Bao, H. Copper-Catalyzed Enantioselective Radical 1,4-Difunctionalization of 1,3-Enynes. J. Am. Chem. Soc. 2020, 142, 18014–18021.
- 20(a) Chen, Y.; Wang, J.; Lu, Y. Decarboxylative 1,4-Carbocyanation of 1,3-Enynes to Access Tetra-Substituted Allenes via Copper/Photoredox Dual Catalysis. Chem. Sci. 2021, 12, 11316–11321; (b) Chen, Y.; Zhu, K.; Huang, Q.; Lu, Y. Regiodivergent Sulfonylarylation of 1,3-Enynes via Nickel/Photoredox Dual Catalysis. Chem. Sci. 2021, 12, 13564–13571; (c) Wang, Q.; Chen, Y.; Peng, K.; Li, Y.; Cheng, L.; Deng, G.-J. Three-Component Cross-Electrophile 1,4-Alkylarylation of 1,3-Enynes by Merging Nickel and Photoredox Catalysis. Org. Lett. 2023, 25, 8889–8894.




