Journal list menu
Export Citations
Download PDFs
Table of Contents
Regioselective Electrochemical Hydroalkylations of [60]Fullerene-Fused Furochromenone
- First Published: 28 November 2022
![Regioselective Electrochemical Hydroalkylations of
[60]Fullerene-Fused Furochromenone](/cms/asset/9e592fe4-9e56-4302-9aad-1cf94ba48db8/cjoc202200669-toc-0001-m.jpg)
Regioselective electrochemical hydroalkylations of [60]fullerene-fused furochromenone with alky halides in the presence of TFA or AcOH unexpectedly afford three types of tetra-functionalized [60]fullerene derivatives in high yields. The usage of AcOH and TFA predominantly gives 1,2,3,4-adducts with the five-membered heterocycle retained at [6,6]-junction and 1,2,3,16-adducts with the five-membered heterocycle rearranged to [5,6]-junction, respectively. The obtained 1,2,3,16-adducts can be transformed into 1,2,3,4-adducts by treatment of NaH followed by AcOH.
Synthesis of Open-Cage Fullerene Derivatives Containing Multiple Hydroxyl and Amino Groups on the Rim of the Orifice
- First Published: 17 January 2023
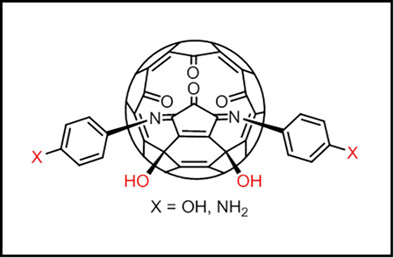
Open-cage C60 derivatives were prepared containing a OH or NH2 on each of the two phenyl rings above the rim of the orifice. These amino and hydroxyl groups on the phenyl ring showed similar reactivity as aniline and phenol, thus acting as a useful handle for attaching other functional groups and also for making polymers containing open-cage fragments.
Cu(OAc)2-Mediated Synthesis of Fullerodihydropyridine-3-ones via the Reaction of [60]Fullerene with β-Substituted Ethylamines in the Absence or Presence of Arylacetaldehydes†
- First Published: 03 March 2023
![Cu(OAc)2-Mediated Synthesis of Fullerodihydropyridine-3-ones via the Reaction of [60]Fullerene with β-Substituted Ethylamines in the Absence or Presence of Arylacetaldehydes†](/cms/asset/f5a43483-c223-4635-ba37-4fbe4d6c38a4/cjoc202200717-toc-0001-m.jpg)
Reaction of [60]fullerene with cheap and readily available β-substituted ethylamines in the absence or presence of arylacetaldehydes under the assistance of Cu(OAc)2 afforded a series of unreported fullerodihydropyridine-3-ones as a new family of fullerene derivatives, which may have promising applications in photovoltaic devices due to the existence of a large π-conjugated system on the dihydropyridine-3-one ring.
Synthesis of [60]Fullerene-Fused Allylbenzofurans via Palladium-Catalyzed Migration Reaction
- First Published: 19 March 2023
![Synthesis of [60]Fullerene-Fused Allylbenzofurans via Palladium-Catalyzed Migration Reaction†](/cms/asset/7ab80535-4852-4343-8f94-84bc8c43adcd/cjoc202300046-toc-0001-m.jpg)
An unprecedented Pd-catalyzed migration reaction of [60]fullerene with allyloxy-tethered aryl iodides is presented for the preparation of rare [60]fullerene-fused allylbenzofurans. The reaction features high chemo- and regio-selectivity. This transformation proceeds via a sequential C—O bond cleavage/allyl-migration/intermolecular cycloaddition cascade process.
ScY@C3v(8)-C82: Metal-Metal σ2 Bond in Mixed Rare-Earth Di-metallofullerenes
- First Published: 14 March 2023
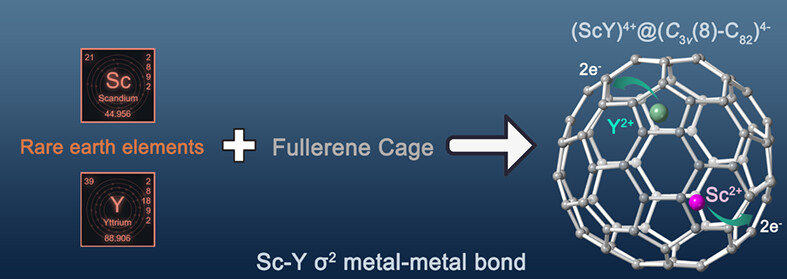
A heteronuclear di-metallofullerene, ScY@C3v(8)-C82, which contains a mixed rare-earth metal-metal bond, was successfully synthesized and characterized. The combined experimental and theoretical results confirm that both Sc and Y atoms transfer two electrons to the C3v(8)-C82 cage. In particular, a covalent Sc-Y σ2 bond, which has never been reported before, is proven to be formed inside C3v(8)-C82 fullerene cage.
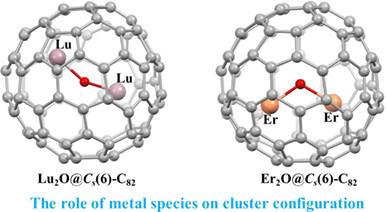
Crystallographic Characterization of Lu2O@Cs(6)-C82 and Er2O@Cs(6)-C82: The Role of Metal Species on Cluster Configuration
- First Published: 04 April 2023
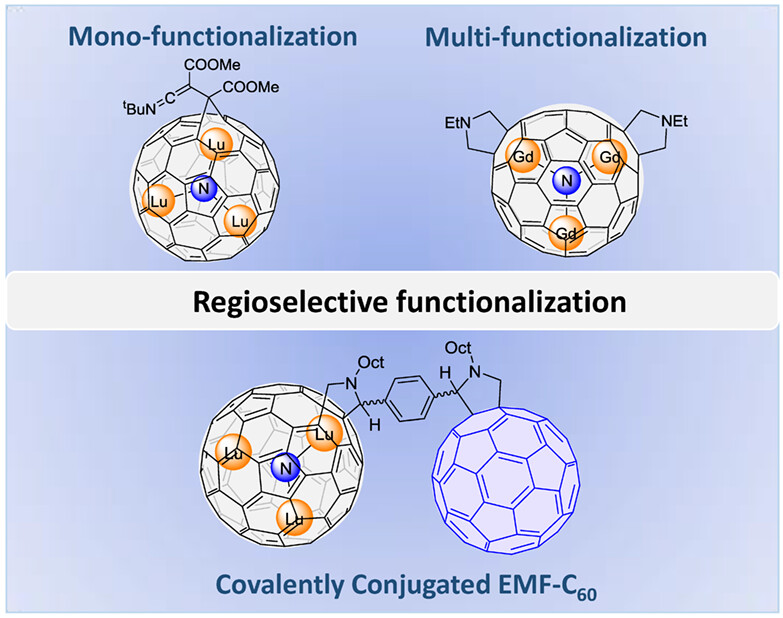
Advances in Regioselective Functionalization of Endohedral Metallofullerenes
- First Published: 31 March 2023
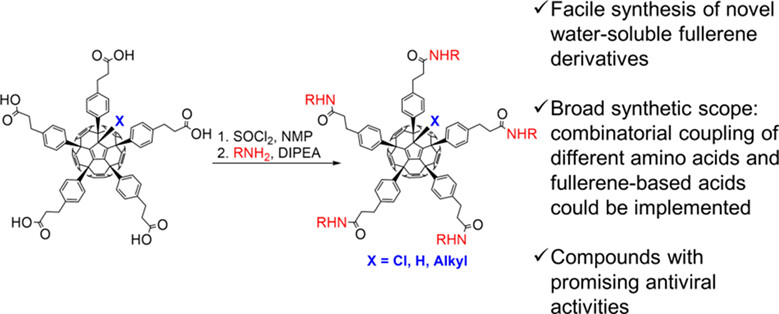
Facile Synthesis of Amino Acid Decorated Water-Soluble Fullerene Derivatives with Anti-influenza Activity
- First Published: 17 March 2023
Stereoselective Synthesis of Nontethered trans-4 Bis(aziridino)[60]fullerene Derivatives
- First Published: 26 April 2023
![Stereoselective Synthesis of Nontethered trans-4 Bis(aziridino)[60]fullerene Derivatives
†](/cms/asset/f6eb48eb-78bc-4370-89f5-bf0a6e9b63dd/cjoc202300094-toc-0001-m.jpg)
We investigated the important role of C60Br8 in the stereoselective synthesis of fullerene bis-adducts using the reaction of C60Br8 with alkoxyaniline, and surprisingly prepared a sequence of nontethered trans-4 bis(aziridino)[60]fullerene with superior stereoselectivity. To the best of our knowledge, the controllable synthesis of non-tethered bis(aziridino)[60]fullerene with high stereoselectivity has never been reported.
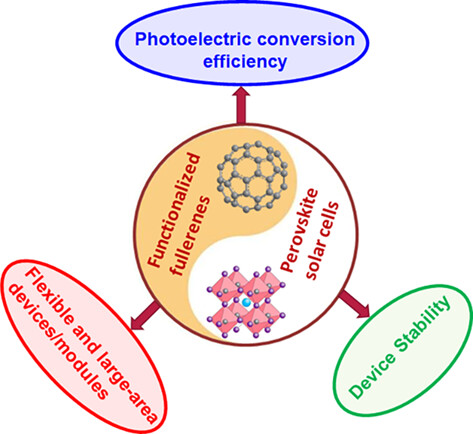
Recent Advances in Functionalized Fullerenes in Perovskite Solar Cells
- First Published: 09 May 2023





![Synthesis of Diverse 1,4-(Azaindole)[60]fullerenes via Transition-Metal Free Three-Component Coupling Reaction of Azaindoles, C60, and Bromoalkanes/Triphenylamines†](/cms/asset/426bc5fa-06ff-4032-b794-3b43c2954449/cjoc202300292-toc-0001-m.jpg)




