Advances in Regioselective Functionalization of Endohedral Metallofullerenes†
Dedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Comprehensive Summary
Endohedral metallofullerenes (EMFs) are a family of molecules with metal clusters or ions encapsulated inside a fullerene cage. Their unique structures provide a powerful interface for organic chemists to manipulate metal elements. Over a decade ago, many reactions on empty fullerenes were adopted for EMFs, resulting in a series of reactions that are well-established and reviewed in the literature. In recent years, new topics have emerged in EMF chemistry. First, new addition reactions were developed, notably some that are not based on known empty-fullerene reactions. Second, considerable progress has been made on regioselective multi-additions on EMFs. Finally, EMFs have been covalently conjugated with fullerene C60 to form functional materials. This Recent Advances article will focus on these new developments and discuss their implication for future directions of EMFs.
1 Introduction
Endohedral metallofullerenes (EMFs),[1-9] with metal atoms or metallic clusters encapsulated in a carbon cage, are a unique class of molecularly precise nanomaterials. Their fascinating electronic structures, resulting from the mutual stabilization of the metal- containing clusters and the carbon cages, led to potential applications in solar cells, energy storage and conversion, molecular electronics, biochemistry, medicine, and nanoscience.[1-8, 10] Meanwhile, the exohedral functionalization of EMFs is essential for the development of novel metallofullerene materials with better solubility, tunable electronic properties, and control over the motion of the endohedral species, as well as added functions from the exohedral appendants, laying a foundation for studying structure-reactivity relationships[7] and broadening the scope of applications.[2]
Compared with empty fullerenes such as C60 and C70,[3, 8] EMFs are less reactive, as the surface of the EMF cage is more electron-rich due to electron transfer from the metal cluster.[1, 2] Additionally, the chemical properties of EMFs are highly dependent on the encapsulated metallic species. Only a small fraction of reactions reported for empty fullerenes[6] have been successfully adopted for EMFs, some of which, however, afforded unexpected product distributions.[5] Several excellent recent reviews[2, 8, 9] have summarized the chemical functionalization of EMFs, including silylation and germylation, Diels–Alder reactions, Prato reactions, Bingel–Hirsch reactions, carbene reactions, diazo additions, benzyne additions, radical additions, reactions with azides, reactions with special 1,3-dipoles, coordination, dimerization and EMF anion-induced reactions. Instead of a comprehensive review, this Recent Advances article will focus on lately emerging topics in EMF chemistry, including three sections: 1) an update of new mono-addition reactions that were reported in the past 3—4 years and have not been well-covered in previous reviews; 2) regioselective multi-additions; and 3) covalent EMF-C60 conjugates.
2 Mono-addition of EMFs
In recent 3—4 years, there have been important updates in the chemical modification of EMFs, mainly including metal clusters or ions encapsulated in C80, C78 and C82 cages. These advances are discussed herein in the order of the EMF cage size and symmetry.
The nitride clusterfullerenes (NCFs), often referred as the trimetallic nitride template (TNT) EMFs with an M3N cluster, are an abundant family of cluster EMFs.[10] In NCFs, six extra electrons donated by the M3N cluster stabilize the fullerene cage, but meanwhile make chemical modification difficult. Among NCFs, functionalization of M3N@Ih-C80 (shortened as M3N@C80 hereafter as the D5h isomer is not discussed in this review) has been widely studied due to the high production yield of the EMF starting materials, while the investigation of other family members, such as M3N@C78, represents challenging yet important work that are in high demand.
Lu3N@C80 is an abundant EMF in the electric-arc synthesis. In 2021, our group in collaboration with Poblet and Rodríguez-Fortea et al. reported one multicomponent reaction involving tert-butyl isocyanide, terminal or internal alkynes, and Lu3N@C80 yielding [6,6]-open metallofulleroids 2a—c and 4 selectively (Scheme 1a).[11] In this process, 1,3-dipolar or 1,4-dipolar species, derived from the interaction between tert-butyl isocyanide and alkynes, are captured by EMF cage. One molecule of H2O gets involved in the formation of 2a—c since the corresponding intermediate ketenimine groups are not as stable as those in 4. It is worth noting that the cluster motion in crystals of 4 has been slowed down, but not in 2a, due to the close interaction with the exohedral organic appendant of a neighboring molecule as supported by DFT calculations. In addition, allenes linked to ester group(s), which are also sp-hybridized, have been taken into consideration,[12] but similar multi-component reactions suffered from low conversion and poor selectivity. Notably, similar reactions[13] have been applied to C60, leading to selective formation of cyclo-adducts for internal alkyne and allene substrates, but no isolable products for terminal alkyne substrates.
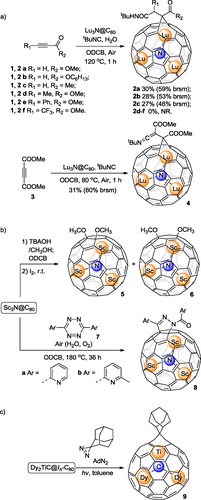
Sc3N@C80 is the first discovered clusterfullerene[14] and a classical example of abundant but chemically inert NCF species. Lu and coworkers reported the alkoxylation[15] of Sc3N@C80 to afford 1,4- and 1,2-bismethoxyl adducts 5 and 6 (Scheme 1b), in which Sc3N@C80 was treated with a methanol solution of tetrabutylammonium hydroxide (TBAOH) and iodine stepwise. Although two methoxy groups are added, this reaction is categorized in this monoaddition section, because the overall degree of unsaturation is reduced by one, while two sp3 carbon atoms are formed on the cage, after the reaction, equivalent to a typical mono-cycloaddition. Both products 5 and 6 were unambiguously characterized by single crystal structures. Generally, a strong base such as NaOH containing TBAOH as a catalyst can be used to install hydroxyl groups on the surface of fullerenes. However, in this case, TBAOH (1.0 M in CH3OH) acted as a deprotonating agent to generate CH3O– from CH3OH in the less polar solvent oDCB, initiating the reaction. Wang, Yang and coworkers reported another unexpected reaction[16] of Sc3N@C80 with 3,6-di(pyridin-2-yl)-1,2,4,5-tetrazines 7, water, and oxygen to form the pyrazoline-fused compounds 8, rather than the expected bis-fulleroid derivatives (Scheme 1b). The multicomponent cascade reaction is complicated, featuring a sequence of Diels-Alder, retro Diels-Alder with N2 extrusion, hydration, rearrangement, and oxidative dehydrogenation reactions.
The μ3-carbido clusterfullerene (abbreviated as μ3-CCF) was discovered in 2014, encapsulating a special cluster in which a central μ3-C atom bonds with a valence IV metal such as titanium (Ti) via a Ti=C double bond along with two rare earth metals.[17] Ti-based μ3-CCFs TiM2C@Ih-C80 (M = Sc, Y, Nd, Gd, Tb, Dy, Er, and Lu) have been isolated, featuring the electronic configuration of [Ti4+(M3+)2C4−]6+@C806− which can be viewed as an isoelectric variation of M3N@C80. The chemical properties of these EMFs, specifically, the impact of the encapsulated Ti4+ cation, remained unknown until the recent report[18] by Yang, Wang, Chen and coworkers. A well-established 2-adamantane-2,3-[3H]-diazirine (abbreviated as AdN2)-involved cycloaddition reaction has been applied to chemically functionalize Dy2TiC@Ih-C80 μ3-CCF (Scheme 1c). Only one [6,6]-open mono-adduct 9, in which the Ad moiety was installed on a [6,6] bond adjacent to the Ti4+ ion instead of the two Dy3+ ions, was formed with high regioselectivity. Theoretical calculations indicated that the Ti(IV) ion plays a decisive role in the high regioselectivity.
EMFs can behave as Lewis bases to react with N-heterocyclic carbenes (NHCs),[19, 20] and the resultant Lewis acid−base adducts exhibit a C—C single bond linking the NHC group and the fullerene cage. Further studies of this reaction were reported by Lu and coworkers in 2019,[21] in which three different lutetium-containing EMFs (Lu3N@Ih(7)-C80, Lu2@C3v(8)-C82, and Lu2@C2v(9)-C82) provided only one monoadduct for each EMF under specific reaction conditions, demonstrating high regioselectivity. Similarly, the regioselective benzyl radical addition to an open-shell metallofullerene also affords the singly bonded EMF derivative.[22-24] In 2022, using this simple benzyl radical functionalization, Yang and Chen[25] captured Dy@C2v(5)-C80, a long-sought dysprosium-based EMF, in the form of Dy@C2v(5)-C80(CH2Ph) from carbon soot containing versatile EMFs.
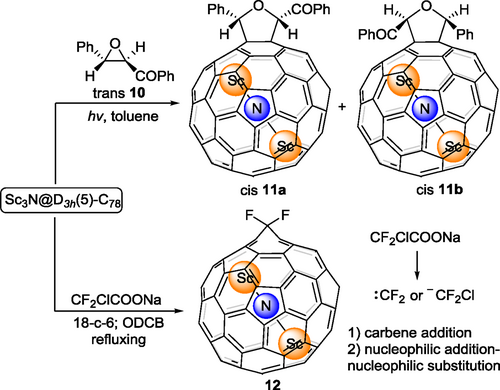
M3N@D3h(5)-C78 is the second most abundant species in the original Sc TNT EMF family.[26] The different cage symmetry leads to different electronic properties and deeply affects the dynamics of the endohedral M3N. For example, in Sc3N@C78, the encaged Sc3N cluster is restricted in the horizontal plane of the ellipsoidal D3h(5)-C78 cage, while for the analogue Sc3N@C80, the same cluster rotates freely. Unlike the well-studied Sc3N@C80, chemical modification of Sc3N@C78 has been scarcely reported because of the less-symmetric cage causing challenging regioselectivity issues. In 2021, Yang, Wang and coworkers reported[27] the synthesis of two Sc3N@C78 monoadducts 11a and 11b, with a [6,6]-closed addition pattern, bearing anomalous cis-conformation regioselectivity (Scheme 2). Under irradiation, the precursor trans 10 could be converted into a carbonyl ylide, which underwent a 1,3-dipolar reaction with Sc3N@C78. Under the same conditions, C60 reacted with trans 10 to generate two isomeric monoadducts sharing cis- and trans-conformations, respectively. Later, in 2022, Popov, Ioffe and coworkers[28] reported the CF2 insertion reaction at the same [6,6] bond of Sc3N@C78. Sodium chlorodifluoroacetate reacted with Sc3N@C78 in the presence of 18-crown-6 to prepare 12 selectively. There could be two CF2 addition pathways: a) carbene addition; b) nucleophilic addition followed by intramolecular nucleophilic substitution. It is challenging to determine which one is more favorable with theoretical calculations.
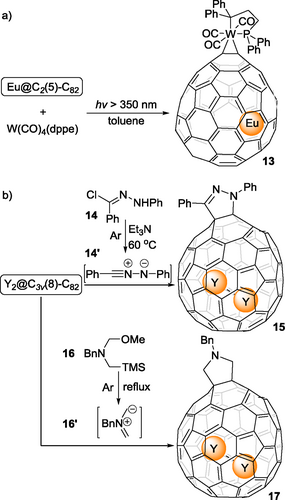
The EMFs with C82 cages show diversity due to the different encapsulated species and different symmetry of the outer cages. In 2019, Lu, Jin and coworkers reported the complexation of tungsten to Eu@C2(5)-C82.[29] Photo-irradiation on a toluene solution containing Eu@C2(5)-C82 and W(CO)4(Ph2PC2H4PPh2) at room temperature led to the formation of the corresponding complex 13 (Scheme 3a), which represents the first example of a functionalized C2(5)-C82 cage in a highly regioselective manner. Because of electrostatic potential redistribution, the motion of the Eu atom inside the cage is significantly restricted upon exohedral tungsten addition. A similar reaction was applicable to Eu@C2(13)-C84. Later, in 2020, Cong, Lu, Jin and coworkers reported the functionalizetion of another C82 fullerene cage, Y2@C3v(8)-C82.[30] Scheme 3b shows the [3+2] cyclization between 1,3-dipolar species 14’ and 16’, derived from precursors 14 and 16, and Y2@C3v(8)-C82 to prepare 15 and 17, respectively with high regioselectivity. There are also reports[31, 32] about chemical functionalization on Y@Cs(6)-C82 (three isomers), Th@C3v(8)-C82 (three isomers) and U@C2v(9)-C82 (four isomers), indicating relatively high regioselectivity as there are so many possibilities for mono-adducts.
Overall, the selectivity of chemical modification of EMFs depends highly on the symmetry of the carbon cage, the number of electrons transferred from the cluster to the carbon cage and the cluster motion inside. The high symmetry and stability of Ih-C806– cage leads to higher production yield of the starting material and fewer potential regioisomers of the product, but the major challenge in their reaction development is their inert nature that prevents reactions that are successful on alkenes or even fullerenes being applied to EMFs with an Ih-C80 cage. On the other hand, EMFs with lower cage symmetry may be more reactive in organic functionalization; however, their lower availability and more possible regioisomers pose practical challenges in screening for highly regioselective reactions and characterizing of the products. New reactions on EMFs, especially those that have not been demonstrated on empty fullerenes, are still of high demand in the research community.
3 Representative Multi-functionalization of EMFs
Most cases of the reported chemistry of EMFs are about the formation and characterization of mono-adducts, rather than multi-adducts. There are mainly two reasons: i) EMFs show low reactivity compared to empty fullerenes, making it challenging to install more functional groups on the surface; ii) There are many chemically non-equivalent C=C bonds that have similar reactivities, so the regioselectivity of multi-addition reactions is a big challenge. Till now, there have been only a few reports of structurally precise EMF multi-adducts (Table 1), even though the formation of bis- and multi-adducts has been observed in more cases. In this section we discuss multi-functionalization on EMFs based on reaction type.
| EMF | Reaction type | Multi-adduct | MS | 1H NMR | 13C NMR | UV/Vis | X-ray | DFT | Ref |
|---|---|---|---|---|---|---|---|---|---|
| La@C2v-C82 a | [2+2] cycloaddition | Tris | √ | √ | √ | √ | [33] | ||
| La@C2v-C82 a | Bingel-Hirsch reaction | Bis | √ | √ | √ | √ | [35] | ||
| Sc3N@D3h-C78 | Bingel-Hirsch reaction | Bis | √ | √ | √ | √ | √ | [36] | |
| La@C82 a | Carbene reaction | Bis and Tris | √ | [37] | |||||
| La2@D2-C72 | Carbene reaction | Bis | √ | √ | √ | √ | [38] | ||
| La2@Ih-C80 | Carbene reaction | Bis | √ | √ | √ | √ | √ | [39] | |
| Lu3N@Ih-C80 | Carbene reaction | Bis | √ | √ | √ | √ | √ | [40] | |
| La@C82 a | Prato reaction | Bis | √ | √ | [41] | ||||
| Y3N@Ih-C80 | Prato reaction | Bis | √ | √ | √ | √ | √ | [42] | |
| Gd3N@Ih-C80 a | Prato reaction | Bis | √ | √ | √ | √ | [42, 44] | ||
| Sc3N@Ih-C80 and Lu3N@Ih-C80 | Prato reaction | Bis | √ | √ | √ | √ | [43] | ||
| Y3N@Ih-C80 | Prato reaction | Tris and Tetra | √ | √ | √ | √ | √ | [45] | |
| Gd3N@Ih-C80 a | Prato reaction | Tris and Tetra | √ | √ | √ | [45] | |||
| Gd@C2v-C82 a | Multi-amination | Heptakis | √ | √ | √ | √ | [48] | ||
| Gd@C2v-C82 a | Multi-amination | Pentakis and Nonkis | √ | √ | [49] | ||||
| Y2C2@Cs-C82 | Trifluoromethylation | Hexadecakis | √ | √ | √ | [53] | |||
| La@C70 a | Trifluoromethylation | Tris | √ | √ | √ | √ | [55] | ||
| Gd@C60 a | Trifluoromethylation | Tris and Pentakis | √ | √ | √ | √ | [56] | ||
| La@C60 | Trifluoromethylation | Tris and Pentakis | √ | √ | √ | √ | [56] |
- a NMR spectroscopy could not be performed with the presence of paramagnetic metal ions.
3.1 Reaction with benzyne
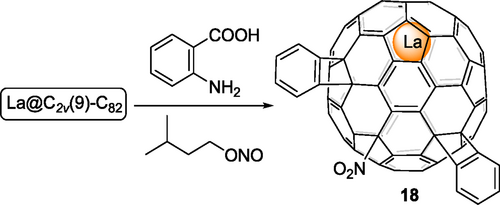
In 2010, Akasaka, Nagase and coworkers first reported the reaction of benzyne and EMFs.[33] The diazotization of anthranilic acid with isoamyl nitrite generates benzyne, which could be captured by La@C2v(9)-C82. There are unavoidable multiple adducts, all of which contain only one NO2 group. Tris-adduct 18 (Scheme 4) with two benzene rings and one NO2 group has been isolated and fully characterized. Later, such a reaction was applied to Sc3N@Ih-C80[34] by Echegoyen, Olmstead, Balch and coworkers. However, only mono [5,6]- and [6,6]-isomers have been fully characterized while MS spectra detected the existence of a series of multi-adducts with up to 10 benzene addends.
3.2 Bingel-Hirsch reaction
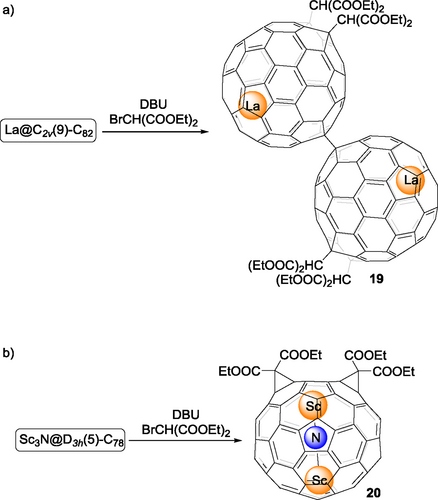
In 2006, Akasaka, Nagase and coworkers reported a well-defined bis-adduct of La@C2v(9)-C82, forming a dimer in its crystalline state.[35] The reaction of La@C2v(9)-C82 with bromomalonate in the presence of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (Bingel-Hirsch reaction) affords the dimer 19 (Scheme 5) in a highly regioselective way and good yield. In 2008, Dorn, Gibson and coworkers[36] carried out a similar Bingel-Hirsch reaction on Sc3N@D3h(5)-C78. Under the condition of lower concentration and a larger excess amount of diethyl bromomalonate, only a monoadduct and the bis-adduct 20 (Scheme 5b) were obtained as the major products (>70%), with relatively minor amounts of the tri-, tetra-, and penta-adducts formed. The novel C2v-adduct 20 represents the first example of a well-studied endohedral metallofullerene bis-adduct, the structure of which has been confirmed by NMR spectroscopy, thanks to its characteristic symmetry, and DFT calculations. It is worth noting that the same cyclopropanation reaction conditions did not work on Sc3N@Ih-C80, clearly indicating that Sc3N@D3h(5)-C78 has significantly higher reactivity than Sc3N@Ih-C80.
3.3 Carbene or carbene analogue reactions
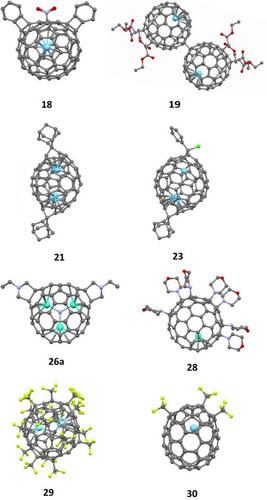
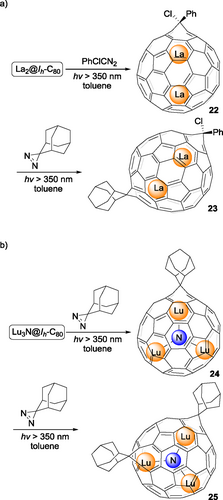
Carbene insertion is one of the earliest-studied reactions on EMFs. However, the formation of multi-adducts has been rarely reported. In 1995, Kato, Suzuki and coworkers[37] reported the reaction between diphenyldiazomethane (Ph2CN2) and La@C82 to afford mono-, bis- and tris-adducts based on the FAB mass spectrum. Unfortunately, due to the limited amount of EMF at that time, these adducts were not fully isolated and characterized. Not until 2008 Nagase, Akasaka and coworkers[38] reported the first example of a bis-adduct of a dimetallic EMF. La2@C72, a D2-symmetric non-isolated-pentagon rule (non-IPR) cage with two pairs of fused pentagons, was treated with AdN2 under similar irradiation conditions described in Scheme 1c to generate seven isomers of the bis-adduct La2@C72Ad2. One of the most abundant isomers 21 was isolated and characterized by single-crystal XRD analysis (as shown in Figure 1), in which two Ad groups were installed at both poles of La2@C72, facing the encaged La atoms. Unlike La2@C72, carbene insertion reaction of Ih-C80 fullerenes with encapsulated metal clusters or ions, including M2@Ih-C80 and M3N@Ih-C80, is challenging due to the free rotation of the inside cluster and the higher symmetry of the cage. A stepwise addition reaction protocol was adopted for regioselective bis-functionalization of La2@Ih-C80[39] and Lu3N@Ih-C80[40] (Scheme 6) by Akasaka, Nagase and coworkers. The photochemical reaction of La2@Ih-C80[39] with phenylchlorodiazirine (PhClCN2) afforded mono-adduct 22 with a [6,6]-open structure, which further reacted with AdN2 under similar irradiation conditions to generate three isomers of the bis-adduct La2@Ih-C80(CClPh)(Ad). One of them has been fully characterized by single-crystal XRD analysis as 23 in Scheme 6a. Similar to the case of La2@Ih-C80,[40] the first reaction of Lu3N@Ih-C80 with AdN2 gives the major mono-adduct [6,6]-open 24 with the minor [5,6]-open isomer. Further reaction of 24 and AdN2 was carried out smoothly to generate two bis-adducts selectively, one of which was fully characterized as 25 in Scheme 6b. In both crystal structures of 23 and 25, the two exohedral groups were attached at a [6,6] bond near a La/Lu atom in a [6,6]-open fashion, indicating that the orientation of inside metal species could regulate the regioselectivity of multiple addition reactions on EMFs to some extent.
3.4 Prato reaction
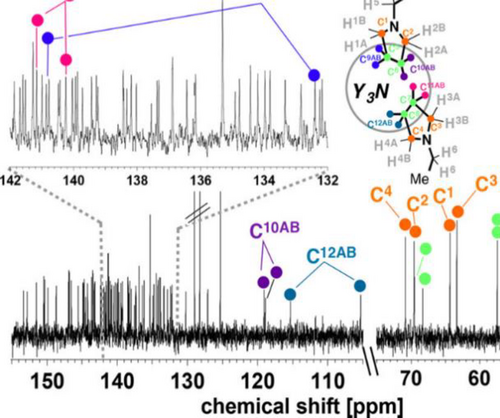
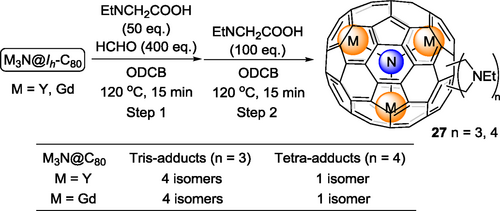
The Prato reaction, one of the most widely-used reactions in fullerene chemistry, in which a newly-formed 1,3-dipolar azomethine ylide undergoes [3+2] cycloaddition with a C=C double bond on the cage, was also adopted to prepare multi-adducts of EMFs. In the first Prato reaction on an EMF reported by Akasaka et al.,[41] La@C82-A reacted with an azomethine ylide to generate the mono-adduct and bis-adduct in up to 99% conversion. Unfortunately, isomers of bis-adducts seen in the HPLC chromatogram, one of which was isolated, were not further characterized due to the limited amount of sample. In 2015, Yamakoshi and coworkers reported the regioselective bis-addition of M3N@C80 (M = Y, Gd),[42] and Echegoyen and coworkers reported the regioselective bis-addition on and M3N@C80 (M = Sc, Lu)[43] independently using the Prato reaction. The structural information of the obtained bis-adducts 26 from both papers is combined in Scheme 7. All structure assignments were made mainly by NMR spectroscopy, UV-vis spectroscopy and DFT calculations. The 13C NMR spectrum of the Y3N@C80 major bis-adduct (Figure 2) in Yamakoshi's work was one key piece of data, based on which the addition sites were confirmed to be on [6,6] junctions. Later, Yamakoshi[44] reported the X-ray structure of a Gd3N@C80 bis-adduct 26a (Scheme 7), which is the minor C2-symmetric [6,6][6,6] isomer. The Gd3N cluster was flattened out in the crystal structure of 26a, because the locally available endohedral space of 26a increased allowing it to stretch out. In 2020, Yamakoshi, Osuna and coworkers[45] reported the formation and isolation of tris- and tetrakis-adducts of M3N@C80 (M = Y, Gd) in a highly regioselective manner using the stepwise Prato reaction strategy (Scheme 8). There were four isolated isomers for tris-adducts and only one isomer for the tetra-adduct, although their crystal structures are yet to be obtained. The electronic paramagnetic resonance (EPR) spectra for the tris-adducts show a broader and relatively weaker EPR signal, suggesting exo-functionalization leads to deactivation of the Gd3N cluster rotational tumbling at ambient temperature.

3.5 Multi-amination reaction
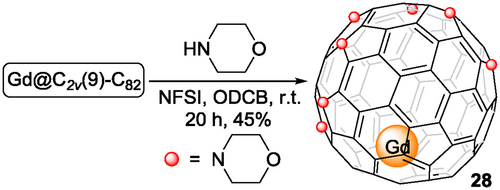
The N-fluorobenzenesulfonimide (NFSI)-mediated multi-amination reaction was established by Gan to achieve the regioselective addition of secondary amines to empty fullerenes, such as C60[46] and C70.[47] This reaction was adopted to functionalize Gd@C2v(9)-C82 (Scheme 9) by Sun, Gao and coworkers[48] in 2018. Gd@C82 was treated with 30 equivalents of morpholine in the presence of NFSI (5 equiv) to form Gd@C82(morpholine)7 28, with seven morpholine groups added to the carbon cage in a 1,4-addition pattern, which was purified using flash chromatography on a silica gel column and fully characterized by single-crystal XRD analysis. Later, Gd@C82(morpholine)n (n = 5 and 9)[49] were isolated from the amination reaction.
3.6 Trifluoromethylation
Trifluoromethylation on C60 and C70 has been well established[50] to prepare so-called perfluoroalkylfullerenes. The trifluoromethylation of EMFs[50] is a typical radical addition reaction. Generally, the addition pattern depends on the size of the fullerene cage and the inner metal clusters or ions. This reaction can be used for the extraction of EMFs, especially unstable ones. The added exohedral CF3 groups make the corresponding EMFs more stable and more soluble in common organic solvents. Gaseous CF3I[50] in quartz ampules at high temperature (e.g., 450 °C) could generally serve as the source of the CF3 radical. The Yang group has used this method to isolate and confirm the structures of yttrium cyanide cluster fullerenes YCN@C82(6),[51] YCN@C84-(23) and YCN@C84(13).[52] Later, Yang and coworkers[53] reported the isolation and unambiguous structural determination of four isomers of yttrium (Y)-based dimetallic carbide clusterfullerene derivatives Y2C2@C82(6)(CF3)16 (29 as an example in Figure 1). Different addition patterns of the 16 CF3 groups lead to different geometries of the Y2C2 cluster within the same Cs-C82(6) carbon cage. Polytetrafluoroethylene (PTFE), an alternative of CF3I, could also be used as a source for the CF3 groups. PTFE, placed near the arc discharge area, is evaporated together with the metal/graphite rod during arc discharge, resulting in a series of trifluoromethyl derivatives of EMFs.[54] This in situ trifluoromethylation method was used by Shinohara and coworkers[55] for the extraction and purification of the missing small-bandgap metallofullerenes including M@C60 and M@C70. Two isomers of La@C70(CF3)3 were obtained, and the structure of one of them 30 was determined by single-crystal X-ray diffraction. Later, one isomer La@C60(CF3)5, and three isomers of Gd@C60(CF3)5[56] were isolated and characterized by single-crystal X-ray diffraction.
4 Covalently Linked EMF-C60 Functional Materials
Multi-fullerene conjugates are a powerful way of constructing molecularly precise nanomaterials from bottom-up. As a remarkable representative, using the hexakis adducts of fullerene C60,[57] Martín and coworkers developed a series of 13-buckyball giant molecules with unique potentials as biomedical and energy materials.[58-60]
In contrast, covalently conjugated EMF-C60 molecules have rarely been achieved. In 2017, Hirsch and coworkers[61] reported the synthesis of an unprecedented hybrid dumbbell consisting of Sc3N@C80 and C60 (Scheme 10). First of all, a [5 : 1] hexakis [60]fullerene adduct with one terminal alkynyl group was synthesized by well-designed stepwise Bingel-Hirsch reactions. The second step was the synthesis of an azido malonate monoadduct of Sc3N@C80 containing one terminal azido group via a Bingel-Hirsch reaction. The copper-catalyzed click reaction between the two precursors gave 31 in 45% isolated yield, the structure of which was characterized by MALDI-TOF mass spectrometry and 1H NMR spectroscopy.

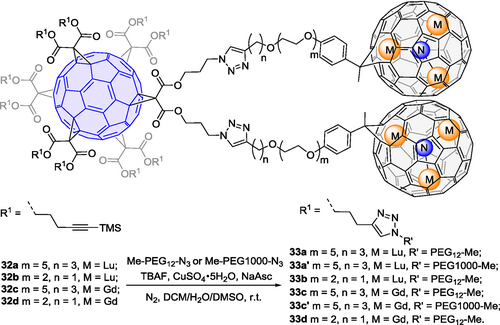
EMFs have shown promise as a great platform for the development of rare-earth element-based metallodrugs, such as (gadolinium-based) MRI contrast agents.[62] A fundamental advantage of EMFs in this regard is that they can definitively seal the toxic heavy metal ions inside the cage, avoiding safety issues of metal leakage. However, most approaches to synthesize water-soluble EMF derivatives focus on introducing a large number of small hydrophilic groups (e.g., -OH, -COOH, -NH2), yielding mixtures. Recently, using the hexakis-C60-based strategy, our group[63] reported the synthesis of a “metallobuckytrio” (MBT) 32 (Scheme 11), a three-buckyball system, as a modular platform to develop structurally-defined water-soluble EMF derivatives with ligands by choice. Like the synthesis of 31, first of all, two click reaction partners were designed and synthesized. The [5 : 1] hexakis [60]fullerene adduct was synthesized by two-step Bingel-Hirsch reactions with two terminal azido groups installed firstly and followed by ten terminal TMS-protected alkynyl groups. Diazo addition reactions were used to functionalize EMFs Lu3N@C80 or Gd3N@C80 with PEG-chain-linked terminal alkynyl groups. The Cu-catalyzed click reaction of the two partners then afforded the corresponding MBT 32 in 59%—93% yield. The structure of 32 was characterized by MALDI-TOF mass spectrometry (for 32a—d) and 1H NMR, 13C NMR spectroscopy (only for 32a—b). A one pot reaction of 32 combining TMS group removal and a click reaction with PEG ligand with various lengths yielded water-soluble MBTs 33 (Scheme 11). These MBTs show superb biocompatibility based on cell viability tests, and the Gd MBTs exhibit superior T1 relaxivity relative to typical Gd complexes, potentially exceeding current clinical MRI contrast agents in both safety and efficiency. Because the MBTs brought the EMF cage to aqueous phase without breaking its conjugation, they are also promising photosensitizers for photodynamic therapy.
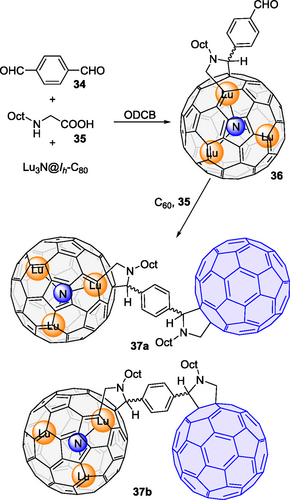
In addition to click chemistry, the EMF-C60 connection was also achieved by Martín, Guldi, Solà and coworkers[64] with an impressive straightforward approach (Scheme 12). The Prato reaction of terephthalaldehyde 34, N-octylglycine 35 and Lu3N@C80 yielded aldehyde 36, which underwent a second Prato reaction with 35 and C60, affording dumbbells 37a and 37b. It was interesting to note that switching the sequence of the two Prato reactions failed to give the desired dumbbells. The structure of 37a/37b was confirmed by MALDI-TOF MS, UV-vis, HPLC, 1H NMR, COSY 1H-1H-NMR, HSQC NMR spectroscopy. However, 37a/37b possesses four chiral centers - two at the nitrogen atoms and two at the C2 position of the pyrrolidine rings, theoretically generating 16 stereoisomers. Additionally, a syn- or an anti-isomer might be generated depending on the relative orientation of C60 and Lu3N@C80 with respect to the plane of the phenyl linker. Therefore, the theoretical maximum number of isomers is 32. DFT calculations were employed to determine the most likely isomers. Notably, due to the higher work functions of Lu3N@C80 than C60, this dyad marked the first all-fullerene electron donor-acceptor conjugate. Femtosecond transient absorption experiments indicated the rapid formation of a C60·–-Lu3N@C80·+ charge-separated state in 5 ps. This state required two lifetime components of 1.2 ns and 8.5 ns, but only a single species-associated absorption spectrum, to model its decay to C60-3Lu3N@C80*. The authors suggested that coexisting singlet and triplet charge-separated states are responsible for this phenomenon.
5 Conclusions and Perspectives
This Recent Advances article provided an overview of new topics emerging in EMF chemistry including recent mono-functionalization of EMFs, regioselective multi-functionalization of EMFs and the synthesis of covalently conjugated EMF-C60 functional molecules. There is still great interest to develop new EMF chemistry, with many important tasks yet to be realized for exciting new materials, such as new reactions that eventually lead to multi-step orifice opening or molecular surgery, regioselective multi-additions like the hexakis Bingel-Hirsch reaction realized on C60, and chemical conversion of a carbon cage to a diamagnetic heterofullerene cage with tunable metal-cage interactions. Meeting these goals will open up new materials horizons in energy, catalysis, and biomedical applications with EMFs being a key elementary building block to achieve functions that cannot be realized with empty fullerenes
Acknowledgement
J.Z. would like to acknowledge the support of the US Department of Energy (Grant No. DE-SC0020260).
Biographies

Yanbang Li was born and grew up in Shandong Province, China. He obtained his B.S. from Shandong University in 2012, and Ph.D from Peking University in 2017, before he became a postdoctoral scholar at Rutgers, the State University of New Jersey, USA. His research interests focus on synthesis, characterization and properties of carbon materials including fullerene, endohedral metallofullerene and curved conjugated macrocycles.
Yue Sun obtained her bachelor's degree in the Department of Chemistry and Molecular Sciences, Wuhan University in 2018. She is now a Ph.D. candidate in the Department of Chemistry and Chemical Biology, Rutgers University under the supervision of Prof. Jianyuan Zhang. Her research focuses on the functionalization reactions on fullerenes and EMFs and exploration of their properties towards applications.
William Kopcha is a native of Connecticut, USA. He attended the University of Connecticut, from which he received a B. S. in 2008 and an M. S. in 2012, studying the noncovalent interactions of surfactants and carbon nanotubes. After trying careers in teaching and industry, he entered a Ph.D. program at Rutgers, the State University of New Jersey, USA, where he now synthesizes novel fullerene-based structures and studies the photophysical and photochemical interactions of fullerenes with other chromophores, notably porphyrins, using ultrafast spectroscopy techniques.
Jianyuan Zhang received his undergraduate degree from Beijing Normal University. After a Ph.D program studying fullerene and metallofullerenes at Virginia Tech, and two postdoctoral studies at University of Washington and Massachusetts Institute of Technology, he started his independent research at Rutgers University – New Brunswick in 2017. The research of Zhang group includes metallofullerenes for quantum information science, fullerenes and metallofullerenes for biomedical materials, and other nanomaterials.




