Synthesis of Open-Cage Fullerene Derivatives Containing Multiple Hydroxyl and Amino Groups on the Rim of the Orifice†
Xueli Liu
Department of Chemistry, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
Search for more papers by this authorRui Gao
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorZhen Liu
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Jialin Ming
Department of Chemistry, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorYi Qiu
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Jie Su
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Liangbing Gan
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorXueli Liu
Department of Chemistry, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
Search for more papers by this authorRui Gao
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorZhen Liu
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Jialin Ming
Department of Chemistry, College of Chemistry and Chemical Engineering, Inner Mongolia University, Hohhot, Inner Mongolia, 010021 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorYi Qiu
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
Search for more papers by this authorCorresponding Author
Jie Su
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Liangbing Gan
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Bioorganic Chemistry and Molecular Engineering of the Ministry of Education, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorDedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Dedicated to Professor Shigeru Yamago on the occasion of his 60th birthday.
Comprehensive Summary
Most of the known open-cage fullerene derivatives contain carbonyl and other relatively inert groups on the rim of the orifice. It is difficult to rationally design further reactions and attach other functional groups on to these open-cage derivatives. In the present work, two molecules of difunctional 1,4-benzenediamine have been incorporated into the rim of an open-cage C60 derivative through one amino group leaving the other amino group free for further functionalization. The difunctional 4-aminophenol reacted analogously to form an open-cage derivative with a free OH group each on the two phenyl rings. The amino and hydroxyl groups on the phenyl ring above the rim of the orifice showed similar reactivity as aniline and phenol. One of the carbonyl groups on the rim of the orifice could be selectively reduced by NaBH4 and P(OEt)3. The reduction reactions were reversible and the reduced products could be readily converted back to the carbonyl precursor. Thus, this redox process acts as a tool to fine tune the size of the orifice for host-guest studies.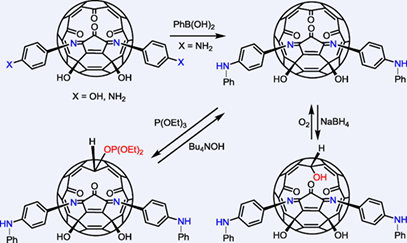
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200829-sup-0001-supinfo.pdfPDF document, 3.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Vougioukalakis, G. C.; Roubelakis, M. M.; Orfanopoulos, M. Open- cage fullerenes: towards the construction of nanosized molecular containers. Chem. Soc. Rev. 2010, 39, 817–844.
- 2 Murata, M.; Murata, Y.; Komatsu, K. Surgery of fullerenes. Chem. Commun. 2008, 6083–6094.
- 3 Gan, L. B.; Yang, D. Z.; Zhang, Q. Y.; Huang, H. Preparation of Open-Cage Fullerenes and Incorporation of Small Molecules through Their Orifices. Adv. Mater. 2010, 22, 1498–1507.
- 4 Shi, L. J.; Gan, L. B. Open-cage fullerenes as tailor-made container for a single water molecule. J. Phys. Org. Chem. 2013, 26, 766–772.
- 5For a recent example of unexpected age opening: Yan, X. X.; Niu, C.; Yin, Z. C.; Lu, W. Q.; Wang, G. W. Anionic alkene-azide cycloaddition (AAAC) strategy toward electrosynthesis of multifunctionalized [60]fullerene derivatives and further applications. Sci. Bull. 2022, 67, 2406–2410.
- 6
Rubin, Y.; Jarrosson, T.; Wang, G. W.; Bartberger, M. D.; Houk, K. N.; Schick, G.; Saunders, M.; Cross, R. J. Insertion of Helium and Molecular Hydrogen through the Orifice of an Open Fullerene. Angew. Chem. Int. Ed. 2001, 40, 1543–1546; Angew. Chem. 2001, 113, 1591–1594.
10.1002/1521-3773(20010417)40:8<1543::AID-ANIE1543>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 7 Iwamatsu, S.; Uozaki, T.; Kobayashi, K.; Re, S.; Nagase, S.; Murata, S. A bowl-shaped fullerene encapsulates a water into the cage. J. Am. Chem. Soc. 2004, 126, 2668–2669.
- 8 Komatsu, K.; Murata, M.; Murata, Y. Encapsulation of Molecular Hydrogen in Fullerene C60 by Organic Synthesis. Science 2005, 307, 238–240.
- 9 Shi, L. J.; Yang, D. Z.; Colombo, F.; Yu, Y. M.; Zhang, W. X.; Gan, L. B. Punching a Carbon Atom of C60 into Its Own Cavity to Form an Endohedral Complex CO@C59O6 under Mild Conditions. Chem. - Eur. J. 2013, 19, 16545–16549.
- 10 Chen, C. S.; Kuo, T. S.; Yeh, W. Y. Encapsulation of Formaldehyde and Hydrogen Cyanide in an Open-Cage Fullerene. Chem. - Eur. J. 2016, 22, 8773–8776.
- 11 Tanaka, T.; Morimoto, K.; Ishida, T.; Takahashi, T.; Fukaya, N.; Choi, J. C.; Kabe, Y. Regioselective Hydroamination of Open-cage Ketolactam Derivatives of C60 with Phenylhydrazine and Water Encapsulation. Chem. Lett. 2018, 47, 503–506.
- 12 Krachmalnicoff, A.; Bounds, R.; Mamone, S.; Alom, S.; Concistrè, M.; Meier, B.; Kouřil, K.; Light, M. E.; Johnson, M. R.; Rols, S.; Horsewill, A. J.; Shugai, A.; Nagel, U.; Rõõm, T.; Carravetta, M.; Levitt, M. H.; Whitby, R. J. The dipolar endofullerene HF@C60. Nat. Chem. 2016, 8, 953–957.
- 13 Hoffman, G.; Bacanu, G. R.; Marsden, E. S.; Walkey, M. C.; Sabba, M.; Bloodworth, S.; Tizzard, G. J.; Levitt, M. H.; Whitby, R. J. Synthesis and 83Kr NMR spectroscopy of Kr@C60. Chem. Commun. 2022, 58, 11284–11287.
- 14 Li, Y. B.; Lou, N.; Xu, D.; Pan, C. W.; Lu, X.; Gan, L. B. Oxygen-Delivery Material: Synthesis of an Open-Cage Fullerene Derivative Suitable for Encapsulation of H2O2 and O2. Angew. Chem. Int. Ed. 2018, 57, 14144–14148.
- 15 Huang, G. L.; Hasegawa, S.; Hashikawa, Y.; Ide, Y.; Hirose, T.; Murata, Y. An H2O2 Molecule Stabilized inside Open-Cage C60 Derivatives by a Hydroxy Stopper. Chem. - Eur. J. 2022, 28, e202103836.
- 16 Sun, S. J.; Liu, Z.; Colombo, F.; Gao, R.; Yu, Y. M.; Qiu, Y.; Su, J.; Gan, L. B. Open-cage Fullerene as Molecular Container for F-, Cl-, Br- and I-. Angew. Chem. Int. Ed. 2022, 61, e202212090.
- 17 Zhang, Q. Y.; Jia, Z. S.; Liu, S. M.; Zhang, G.; Xiao, Z.; Yang, D. Z.; Gan, L. B.; Wang, Z. M.; Li, Y. L., Efficient Cage-Opening Cascade Process for the Preparation of Water-Encapsulated [60]Fullerene Derivatives. Org. Lett. 2009, 11, 2772–2774.
- 18 Gan, L. B. The Chemistry of Fullerene-Mixed Peroxide. Chin. J. Chem. 2018, 36, 991–994.
- 19 Xu, L.; Liang, S. S.; Sun, J. H.; Gan, L. B. Open-cage fullerene with a stopper acts as a molecular vial for a single water molecule. Org. Chem. Front. 2015, 2, 1500–1504.
- 20 Abedinifar, F.; Mahdavi, M.; Rezaei, E. B.; Asadi, M.; Larijani, B. Recent Developments in Arylation of N-Nucleophiles via Chan-Lam Reaction: Updates from 2012 Onwards. Curr. Org. Synth. 2022, 19, 16–30.
- 21 Zhang, H.; Su, J.; Pan, C. W.; Lu, X.; Gan, L. B. Synthesis of an open-cage fullerene-based unidirectional H-bonding network and its coordination with titanium. Org. Chem. Front. 2019, 6, 1397–1402.
- 22 Zhang, Q. Y.; Pankewitz, T.; Liu, S. M.; Klopper, W.; Gan, L. B. Switchable Open-Cage Fullerene for Water Encapsulation. Angew. Chem. Inter. Ed. 2010, 49, 9935–9938.
- 23 Guo, Y.; Yan, J. J.; Khashab, N. M. Conjugation-Promoted Reaction of Open-Cage Fullerene: A Density Functional Theory Study. ChemPhysChem 2012, 13, 751–755.
- 24 Niu, C.; Xu, Z. I.; Huang, X. M.; Wang, W. F.; Yin, Z. C.; Wang, G. W. Electrosynthesis of Decorated Basket Molecules: [60]Fullerene-Fused 12-Membered Macrolactones. Org. Lett. 2022, 24, 5530–5534.
Citing Literature
15 June, 2023
Pages 1471-1477




