Synthesis of [60]Fullerene-Fused Allylbenzofurans via Palladium-Catalyzed Migration Reaction†
Corresponding Author
Tong-Xin Liu
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected] or [email protected] or [email protected]Search for more papers by this authorPanting Yang
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorNana Ma
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorXiaojun Li
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorXin Wang
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorJinliang Ma
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorCorresponding Author
Guisheng Zhang
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected] or [email protected] or [email protected]Search for more papers by this authorCorresponding Author
Tong-Xin Liu
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected] or [email protected] or [email protected]Search for more papers by this authorPanting Yang
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorNana Ma
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorXiaojun Li
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorXin Wang
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorJinliang Ma
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
Search for more papers by this authorCorresponding Author
Guisheng Zhang
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected] or [email protected] or [email protected]Search for more papers by this authorDedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Comprehensive Summary
Chemical functionalization of fullerenes has been an important topic in fullerene chemistry. Herein, an unprecedented Pd-catalyzed migration reaction of [60]fullerene with allyloxy-tethered aryl iodides is present for the preparation of novel [60]fullerene-fused allylbenzofurans. The use of 1,2-bis(diphenylphosphino)benzene (DPPBz) as a ligand is crucial for the success of the transformation. The reaction shows high chemo- and regioselectivity, and is flexible with regard to allyl-migration site, providing a new and efficient approach to rare [60]fullerene-fused benzofurans. Control experiments disclose that the reaction most probably undergoes a sequential C—O bond cleavage/allyl-migration/intermolecular cycloaddition cascade process.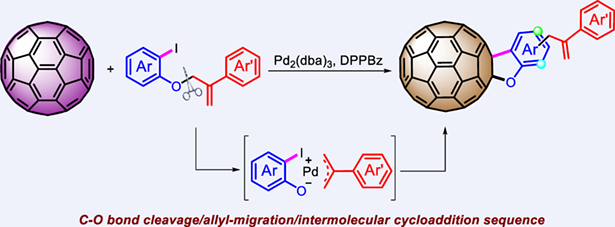
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300046-sup-0001-supinfo.pdfPDF document, 15.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Hirsch, A.; Brettreich, M. Fullerenes: Chemistry and Reactions, Wiley-VCH, Weinheim, Germany, 2005;
10.1002/3527603492 Google Scholar(b) Handbook of Fullerene Science and Technology, Eds.: X. Lu; T. Akasaka; Z. Slanina, Springer, Singapore, 2022.10.1007/978-981-16-8994-9 Google Scholar
- 2(a) Vostrowsky, O.; Hirsch, A. Heterofullerenes. Chem. Rev. 2006, 106, 5191−5207; (b) Matsuo, Y.; Nakamura, E. Selective Multiaddition of Organocopper Reagents to Fullerenes. Chem. Rev. 2008, 108, 3016–3028; (c) Murata, M.; Murata, Y.; Komatsu, K. Surgery of fullerenes. Chem. Commun. 2008, 6083–6094; (d) Gan, L. Molecular Containers Derived from [60]Fullerene through Peroxide Chemistry. Acc. Chem. Res. 2019, 52, 1793–1801; (e) Yamada, M.; Akasaka, T.; Nagase, S. Carbene Additions to Fullerenes. Chem. Rev. 2013, 113, 7209–7264; (f) Tzirakis, M. D.; Orfanopoulos, M. Radical Reactions of Fullerenes: From Synthetic Organic Chemistry to Materials Science and Biology. Chem. Rev. 2013, 113, 5262−5321; (g) Itami, K. Molecular Catalysis for Fullerene Functionalization. Chem. Rec. 2011, 11, 226−235; (h) Wang, G.-W. Functionalization of [60]Fullerene via Palladium-Catalyzed C−H Bond Activation. Top. Organomet. Chem. 2016, 55, 119–136; (i) Maroto, E. E.; Izquierdo, M.; Reboredo, S.; Marco-Martínez, J.; Filippone, S.; Martín, N. Chiral Fullerenes from Asymmetric Catalysis. Acc. Chem. Res. 2014, 47, 2660−2670; (j) Lin, H.-S.; Matsuo, Y. Functionalization of [60]fullerene through fullerene cation intermediates. Chem. Commun. 2018, 54, 11244−11259; (k) Wang, G.-W. Fullerene Mechanochemistry: Serendipitous Discovery of Dumb-Bell-Shaped C120 and Beyond. Chin. J. Chem. 2021, 39, 1797−1803; (l) Niu, C.; Wang, G.-W. Progress in Electrochemical Reactions of [60]Fullerene- Fused Heterocycles. Chin. J. Org. Chem. 2020, 40, 3633−3645; (m) Su, Y.; Chen, Z.-C.; Tian, H.-R.; Xu, Y.-Y.; Zhang, Q.; Xie, S.-Y.; Zheng, L.-S. Implications of Nitrogen Doping on Geometrical and Electronic Structure of the Fullerene Dimers. Chin. J. Chem. 2021, 39, 93–98; (n) Shen, W.; Bao, L.; Lu, X. Endohedral Metallofullerenes: An Ideal Platform of Sub-Nano Chemistry. Chin. J. Chem. 2022, 40, 275–284.
- 3Selected recent works, see: (a) Hashikawa, Y.; Fushino, T.; Murata, Y. Double-Holed Fullerenes. J. Am. Chem. Soc. 2020, 142, 20572−20576; (b) Liu, K.-Q.; Wang, J.-J.; Yan, X.-X.; Niu, C.; Wang, G.-W. Regioselective electrosynthesis of tetra- and hexa-functionalized [60]fullerene derivatives with unprecedented addition pattern. Chem. Sci. 2020, 11, 384−388; (c) Chen, X.-R.; Li, Y.-M.; Li, X.; Xuan, J.; Zhou, H.-P.; Tian, Y.-P.; Li, F. An “umpolung relay” strategy: One-pot, twice polarity inversion cascade synthesis of diversified [60]fulleroindoles. Org. Lett. 2021, 23, 1302−1308; (d) Đorđevic, L.; Casimiro, L.; Demitri, N.; Baroncini, M.; Silvi, S.; Arcudi, F.; Credi, A.; Prato, M. Light-Controlled Regioselective Synthesis of Fullerene Bis-Adducts. Angew. Chem. Int. Ed. 2021, 60, 313−320; (e) Lin, H.-S.; Ma, Y.; Xiang, R.; Manzhos, S.; Jeon, I.; Maruyama, S.; Matsuo, Y. One-step direct oxidation of fullerene-fused alkoxy ethers to ketones for evaporable fullerene derivatives. Commun. Chem. 2021, 4, 74; (f) Ubasart, E.; Borodin, O.; Fuertes-Espinosa, C.; Xu, Y.; García-Simón, C.; Gómez, L.; Juanhuix, J.; Gándara, F.; Imaz, I.; Maspoch, D.; von Delius, M.; Ribas, X. A three- shell supramolecular complex enables the symmetry-mismatched chemo- and regioselective bis-functionalization of C60. Nat. Chem. 2021, 13, 420−427; (g) Zhang, S.; Hashikawa, Y.; Murata, Y. Cage- Expansion of Fullerenes. J. Am. Chem. Soc. 2021, 143, 12450−12454; (h) Ma, J.; Liu, T.-X.; Zhang, P.; Zhao, X.; Zhang, G. Metal-Free-Catalyzed Three-Component [2+2+2] Annulation Reaction of [60]Fullerene, Ketones, and Indoles: Access to Diverse [60]Fullerene-Fused 1,2-Tetrahydrocarbazoles. Org. Lett. 2021, 23, 1775−1781; (i) Niu, C.; Xu, Z.; Huang, X.; Wang, W.-F.; Yin, Z.-C.; Wang, G.-W. Electrosynthesis of Decorated Basket Molecules: [60]Fullerene-Fused 12-Membered Macrolactones. Org. Lett. 2022, 24, 5530−5534; (j) Sun, S.; Liu, Z.; Colombo, F.; Gao, R.; Yu, Y.; Qiu, Y.; Su, J.; Gan, L. Open-Cage Fullerene as Molecular Container for F-, Cl-, Br- and I-. Angew. Chem. Int. Ed. 2022, 61, e202212090; (k) Sun, Y.; Qian, C.; Emge, T. J.; Li, Y.; Kopcha, W. P.; Wang, L.; Zhang, J. Synthesis of [60]- and [70]Fullerene-Fused Tetrahydroquinoxaline Derivatives by Oxidative [4 + 2] Cycloaddition with Unusual Reactivity and Regioselectivity. Org. Lett. 2022, 24, 6417−6422; (l) Liu, T.-X.; Zhu, X.; Xia, S.; Wang, X.; Zhang, P.; Zhang, G. NHC-Catalyzed Three-Component Hydroalkylation Reactions of [60]Fullerene: An Umpolung Approach to Diverse Monoalkylated Hydrofullerenes. Org. Lett. 2022, 24, 3691−3695; (m) Yin, Z.-C.; Niu, C.; Li, M.; Liu, W.-R.; Wang, G.-W. Regioselective Electrochemical Hydroalkylations of [60]Fullerene-Fused Furochromenone. Chin. J. Chem. 2023, 41, 769−775; (n) Yan, X.-X.; Niu, C.; Yin, Z.-C.; Lu, W.-Q.; Wang, G.-W. Anionic alkene-azide cycloaddition (AAAC) strategy toward electrosynthesis of multifunctionalized [60]fullerene derivatives and further applications. Sci. Bull. 2022, 67, 2406−2410.
- 4(a) Lu, S.; Si, W.; Bao, M.; Yamamoto, Y.; Jin, T. Co-Catalyzed Radical Cycloaddition of [60]Fullerene with Active Dibromides: Selective Synthesis of Carbocycle-Fused Fullerene Monoadducts. Org. Lett. 2013, 15, 4030−4033; (b) Si, W.; Zhang, X.; Asao, N.; Yamamoto, Y.; Jin, T. Ni-Catalyzed direct 1,4-difunctionalization of [60]fullerene with benzyl bromides. Chem. Commun. 2015, 51, 6392−6394; (c) Jiang, S.-P.; Su, Y.-T.; Liu, K.-Q.; Wu, Q.-H.; Wang, C.-Y.; Yang, S.; Wang, G.-W. Copper(I)-catalyzed heteroannulation of [60]fullerene with ketoxime acetates: preparation of novel 1-fulleropyrrolines. Chem. Commun. 2015, 51, 6548−6551; (d) Yang, H.-T.; Ge, J.; Lu, X.-W.; Sun, X.-Q.; Miao, C.-B. Copper-Catalyzed Functionalizations of C60 with Amino Alcohols. J. Org. Chem. 2017, 82, 5873−5880; (e) Ueda, M.; Sakaguchi, T.; Hayama, M.; Nakagawa, T.; Matsuo, Y.; Munechika, A.; Yoshida, S.; Yasuda, H.; Ryu, I. Regio- and stereo-selective intermolecular [2+2] cycloaddition of allenol esters with C60 leading to alkylidenecyclobutane-annulated fullerenes. Chem. Commun. 2016, 52, 13175−13178; (f) Liu, T.-X.; Hua, S.; Ma, N.; Zhang, P.; Bi, J.; Zhang, Z.; Zhanga, G. Reactivity and Synthetic Applications of α-Functionalized Oxime Acetates: Divergent Access to Fulleropyrrolidines and Mono- and Disubstituted 1-Fulleropyrrolines via Copper- Catalyzed Redox-Neutral N-Heteroannulation with [60]Fullerene. Adv. Synth. Catal. 2018, 360, 142−152; (g) Liu, T.-X.; Wei, J.; Zhang, P.; Ru, Y.; Ma, J.; Zhang, X.; Ma, N.; Zhang, G. Copper-Catalyzed N−H/C−H Sequential Relay Oxidative Radical Carboannulation: Construction of Diversely Substituted [60]Fullerene-Fused Tetrahydrocyclopenta[b]indoles. Org. Lett. 2019, 21, 6461−6465; (h) Wu, C.; Liu, T.-X.; Zhang, P.; Zhu, X.; Zhang, G. Iron-Catalyzed Redox-Neutral Radical Cascade Reaction of [60]Fullerene with γ,δ-Unsaturated Oxime Esters: Preparation of Free (N−H) Pyrrolidino[2′,3′:1,2]fullerenes. Org. Lett. 2020, 22, 7327−7332.
- 5(a) Zhu, B.; Wang, G.-W. Synthesis of [60]Fulleroindolines: Palladium-Catalyzed Heteroannulations of [60]Fullerene with o-Iodoanilines. J. Org. Chem. 2009, 74, 4426−4428; (b) Hashikawa, Y.; Murata, M.; Wakamiya, A.; Murata, Y. Palladium-Catalyzed Cyclization: Regioselectivity and Structure of Arene-Fused C60 Derivatives. J. Am. Chem. Soc. 2017, 139, 16350−16358; (c) Feng, J.; Wu, Y.; Yu, Q.; Liu, Y.; Jiang, W.; Wang, D.; Wang, Z. Fuller-Rylenes: Cross-Dimensional Molecular Carbons. CCS Chem. 2020, 2, 271−279; (d) Liu, Q.; Liu, T.-X.; Ru, Y.; Zhu, X.; Zhang, G. Palladium-catalyzed decarboxylative heterocyclizations of [60]fullerene: preparation of novel vinyl-substituted [60]fullerene-fused tetrahydrofurans/pyrans/quinolones. Chem. Commun. 2019, 55, 14498−14501; (e) Liu, Q.; Liu, T.-X.; Ma, J.; Zhang, G. Palladium-Catalyzed Three-Component Tandem Coupling Carboannulation Reaction Leading to Polysubstituted [60]Fullerene-Fused Cyclopentanes. Org. Lett. 2020, 22, 284−289; (f) Ma, J.; Liu, T.-X.; Zhang, P.; Zhang, C.; Zhang, G. Palladium-catalyzed domino spirocyclization of [60]fullerene: synthesis of diverse [60]fullerene-fused spiro[4,5]/[5,5] derivatives. Chem. Commun. 2021, 57, 49−52; (g) Liu, T.-X.; Zhang, C.; Zhang, P.; Wang, X.; Ma, J.; Zhang, G. Palladium-catalyzed decarboxylative [2 + 3] cyclocarbonylation reactions of [60]fullerene: selective synthesis of [60]fullerene-fused 3-vinylcyclopentan-4-ones and cyclopentane-4-carbaldehydes. Org. Chem. Front. 2022, 9, 5564−5570; (h) Liu, T.-X.; Wang, X.; Zhang, P.; Yang, P.; Li, X.; Zhang, G. Assembly of Diverse [60]Fullerene-Fused Tricyclic Scaffolds via a Palladium- Catalyzed Cascade [2 + 2 + 2] Annulation Reaction. Org. Lett. 2022, 24, 9102−9106; (i) Su, Y.-T.; Yin, Z.-C.; Wang, G.-W. Palladiumcatalyzed three-component 1,4-aminoarylation of [60]fullerene with aryl iodides and N-methoxysulfonamides, and further transformations. Org. Chem. Front. 2022, 9, 2739−2745; (j) Su, Y.-T.; Yin, Z.-C.; Wang, G.-W. Palladium-Catalyzed Three-Component 1,4-Alkoxyarylation Reaction of [60]Fullerene. J. Org. Chem. 2022, 87, 4051−4060.
- 6(a) Zhou, D.-B.; Wang, G.-W. Synthesis of [60]Fullerene-Fused Spiroindanes by Palladium-Catalyzed Oxidative Annulation of [60]Fullerene with 2-Aryl Cyclic 1,3-Dicarbonyl Compounds. Org. Lett. 2016, 18, 2616−2619; (b) Zhou, D.-B.; Wang, G.-W. Synthesis of [60]Fullerene-Fused Tetralones via Palladium-Catalyzed Ketone-Directed sp2 C−H Activation and sp3 C−H Functionalization. Adv. Synth. Catal. 2016, 358, 1548−1554; (c) Li, F.; Wang, J.-J.; Wang, G.-W. Palladium-catalyzed synthesis of [60]fullerenefused benzofurans via heteroannulation of phenols. Chem. Commun. 2017, 53, 1852−1855; (d) Hussain, M.; Chen, M.; Yang, S.; Wang, G.-W. Palladium-Catalyzed Heteroannulation of Indole-1-carboxamides with [60]Fullerene and Subsequent Electrochemical Transformations. Org. Lett. 2019, 21, 8568−857; (e) Hussain, M.; Niu, C.; Wang, G.-W. Palladium-catalyzed synthesis of [60]fullerene-fused furochromenones and further electrochemical functionalization. Org. Chem. Front. 2020, 7, 1249–1254; (f) Wang, C.; Liu, Z.; Yin, Z.-C.; Wang, G.-W. Synthesis of [60]fullerene-fused dihydrobenzooxazepines via the palladium catalyzed oxime-directed C–H bond activation and subsequent electrochemical functionalization. Org. Chem. Front. 2020, 7, 2518–2525; (g) Liu, Q.-S.; Qiu, W.-J.; Niu, C.; Wang, G.-W. Palladium-Catalyzed C–H Activation/Cyclization for the Synthesis of [60]Fullerene-Fused Phosphinolactones. J. Org. Chem. 2022, 87, 15754−15761.
- 7(a) Maroto, E. E.; Mateos, J.; Garcia-Borràs, M.; Osuna, S.; Filippone, S.; Herranz, M. Á.; Murata, Y.; Solà, M.; Martín, N. Enantiospecific cis−trans Isomerization in Chiral Fulleropyrrolidines: Hydrogen- Bonding Assistance in the Carbanion Stabilization in H2O@C60. J. Am. Chem. Soc. 2015, 137, 1190−1197; (b) Marco-Martínez, J.; Vidal, S.; FernÁndez, I.; Filippone, S.; Martín, N. Stereodivergent-at-Metal Synthesis of [60]Fullerene Hybrids. Angew. Chem. Int. Ed. 2017, 56, 2136–2139; (c) Vidal, S.; Izquierdo, M.; Alom, S.; Garcia Borràs, M.; Filippone, S.; Osuna, S.; Solà, M.; Whitby, R. J.; Martín, N. Effect of incarcerated HF on the exohedral chemical reactivity of HF@C60. Chem. Commun. 2017, 53, 10993–10996; (d) Girón, R. M.; Ouyang, J.; Favereau, L.; Vanthuyne, N.; Crassous, J.; Filippone, S. M.; Martín, N. Reversible Stereodivergent Cycloaddition of Racemic Helicenes to [60]Fullerene: A Chiral Resolution Strategy. Org. Lett. 2018, 20, 1764−1767.
- 8(a) Mehta, V. P.; García-López, J.-A. σ-Alkyl-Pd(II) Species for Remote C-H Functionalization. ChemCatChem 2017, 9, 1149−1156; (b) Ping, Y.; Li, Y.; Zhu, J.; Kong, W. Construction of Quaternary Stereocenters by Palladium-Catalyzed Carbopalladation-Initiated Cascade Reactions. Angew. Chem. Int. Ed. 2019, 58, 1562−1573.
- 9(a) Darwish, A. D.; Avent, A. G.; Kroto, H. W.; Taylor, R.; Walton, D. R. M. Novel base-catalysed formation of benzo[b]furano[60]- and -[70]fullerenes. J. Chem. Soc., Perkin Trans. 2 1999, 1983;
10.1039/a905123f Google Scholar(b) Nambo, M.; Segawa, Y.; Itami, K. Aziridinofullerene: A Versatile Platform for Functionalized Fullerenes. J. Am. Chem. Soc. 2011, 133, 2402−2405.
- 10 Liu, C.; Li, Y.; Shi, W.-Y.; Ding, Y.-N.; Zheng, N.; Liu, H.-C.; Liang, Y.-M. Palladium-Catalyzed Chemoselective Oxidative Addition of Allyloxy- Tethered Aryl Iodides: Synthesis of Medium-Sized Rings and Mechanistic Studies. Org. Lett. 2021, 23, 4311−4316.
- 11(a) Kuram, M. R.; Bhanuchandra, M.; Sahoo, A. K. Direct Access to Benzo[b]furans through Palladium-CatalyzedOxidative Annulation of Phenols and Unactivated Internal Alkynes. Angew. Chem. Int. Ed. 2013, 52, 4607−4612; (b) Sharma, U.; Naveen, T.; Maji, A.; Manna, S.; Maiti, D. Palladium-Catalyzed Synthesis of Benzofurans and Coumarins from Phenols and Olefins. Angew. Chem. Int. Ed. 2013, 52, 12669−12673.
- 12 Wong, W.-Y.; Wang, X.-Z.; He, Z.; Djurišić, A. B.; Yip, C.-T.; Cheung, K.-Y.; Wang, H.; Mak, C. S. K.; Chan, W.-K. Metallated conjugated polymers as a new avenue towards high-efficiency polymer solar cells. Nat. Mater. 2007, 6, 521−527.
Citing Literature
15 July, 2023
Pages 1733-1739




