Facile Synthesis of Amino Acid Decorated Water-Soluble Fullerene Derivatives with Anti-influenza Activity†
Valeriya S. Bolshakova
Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, Academician Semenov ave. 1, Chernogolovka, 142432 Russia
Higher Chemical College of RAS, D.I. Mendeleev University of Chemical Technology of Russia, Miusskaya square 9, Moscow, 125047 Russia
Search for more papers by this authorEkaterina O. Sinegubova
St. Petersburg Pasteur Institute, Mira st. 14, St. Petersburg, 197101 Russia
Search for more papers by this authorYana L. Esaulkova
St. Petersburg Pasteur Institute, Mira st. 14, St. Petersburg, 197101 Russia
Search for more papers by this authorAlexander S. Peregudov
A.N. Nesmeyanov Institute of Organoelement compounds of RAS, Vavylova St. 28, B-334, Moscow, 119991 Russia
Search for more papers by this authorEkaterina A. Khakina
A.N. Nesmeyanov Institute of Organoelement compounds of RAS, Vavylova St. 28, B-334, Moscow, 119991 Russia
National Research University Higher School of Economics, Vavylova St. 7, Moscow, 101000 Russia
Search for more papers by this authorNikita A. Slesarenko
Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, Academician Semenov ave. 1, Chernogolovka, 142432 Russia
Search for more papers by this authorAlexander F. Shestakov
Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, Academician Semenov ave. 1, Chernogolovka, 142432 Russia
Department of Fundamental Physics & Chemical Engineering, M.V. Lomonosov Moscow State University, Leninskie Gory 1/51, Moscow, 119991 Russia
Search for more papers by this authorVladimir V. Zarubaev
St. Petersburg Pasteur Institute, Mira st. 14, St. Petersburg, 197101 Russia
Search for more papers by this authorCorresponding Author
Pavel A. Troshin
Zhengzhou Research Institute, Harbin Institute of Technology, Longyuan East 7th26, Jinshui District, Zhengzhou, Henan, 450003 China
Harbin Institute of Technology, West Dazhi Street 92, Nan Gang District, Harbin, Heilongjiang, 150001 China
Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, Academician Semenov ave. 1, Chernogolovka, 142432 Russia
E-mail: [email protected]Search for more papers by this authorOlga A. Kraevaya
Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, Academician Semenov ave. 1, Chernogolovka, 142432 Russia
Search for more papers by this authorValeriya S. Bolshakova
Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, Academician Semenov ave. 1, Chernogolovka, 142432 Russia
Higher Chemical College of RAS, D.I. Mendeleev University of Chemical Technology of Russia, Miusskaya square 9, Moscow, 125047 Russia
Search for more papers by this authorEkaterina O. Sinegubova
St. Petersburg Pasteur Institute, Mira st. 14, St. Petersburg, 197101 Russia
Search for more papers by this authorYana L. Esaulkova
St. Petersburg Pasteur Institute, Mira st. 14, St. Petersburg, 197101 Russia
Search for more papers by this authorAlexander S. Peregudov
A.N. Nesmeyanov Institute of Organoelement compounds of RAS, Vavylova St. 28, B-334, Moscow, 119991 Russia
Search for more papers by this authorEkaterina A. Khakina
A.N. Nesmeyanov Institute of Organoelement compounds of RAS, Vavylova St. 28, B-334, Moscow, 119991 Russia
National Research University Higher School of Economics, Vavylova St. 7, Moscow, 101000 Russia
Search for more papers by this authorNikita A. Slesarenko
Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, Academician Semenov ave. 1, Chernogolovka, 142432 Russia
Search for more papers by this authorAlexander F. Shestakov
Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, Academician Semenov ave. 1, Chernogolovka, 142432 Russia
Department of Fundamental Physics & Chemical Engineering, M.V. Lomonosov Moscow State University, Leninskie Gory 1/51, Moscow, 119991 Russia
Search for more papers by this authorVladimir V. Zarubaev
St. Petersburg Pasteur Institute, Mira st. 14, St. Petersburg, 197101 Russia
Search for more papers by this authorCorresponding Author
Pavel A. Troshin
Zhengzhou Research Institute, Harbin Institute of Technology, Longyuan East 7th26, Jinshui District, Zhengzhou, Henan, 450003 China
Harbin Institute of Technology, West Dazhi Street 92, Nan Gang District, Harbin, Heilongjiang, 150001 China
Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, Academician Semenov ave. 1, Chernogolovka, 142432 Russia
E-mail: [email protected]Search for more papers by this authorOlga A. Kraevaya
Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, Academician Semenov ave. 1, Chernogolovka, 142432 Russia
Search for more papers by this authorDedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Comprehensive Summary
Herein, we present a facile synthesis of amide-type water-soluble fullerene derivatives decorated with five residues of amino acids. As compared to the previously developed approaches, the proposed method enables usage of a broad range of amino acids, including those unstable under Friedel-Crafts reaction conditions. Furthermore, it is fully tolerant to the nature of the sixth addend X in C60R5X precursor compounds and allows one to obtain fullerene derivatives with X=Cl, H, alkyl. The synthesized amide-type water-soluble fullerene derivatives demonstrated promising antiviral activities against rimantadine-resistant influenza А/Puerto Rico/8/34 (Н1N1) virus in vitro.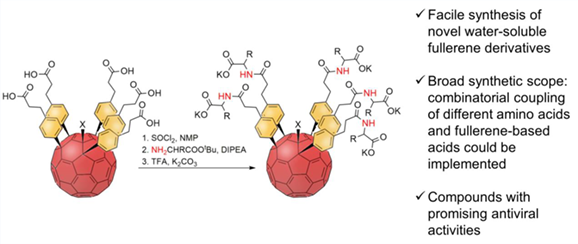
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300050-sup-0001-supinfo.pdfPDF document, 3.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Sharoyko, V. V.; Ageev, S. V.; Podolsky, N. E.; Petrov, A. V.; Litasova, E. V.; Vlasov, T. D.; Vasina, L. V.; Murin, I. V.; Piotrovskiy, L. B.; Semenov, K. N. Biologically active water-soluble fullerene adducts: Das Glasperlenspiel (by H. Hesse)? J. Mol. Liq. 2021, 323, 114990.
- 2 Liu, K.-Q.; Wang, J.-J.; Yan, X.-X.; Niu, C.; Wang, G.-W. Regioselective electrosynthesis of tetra- and hexa-functionalized [60]fullerene derivatives with unprecedented addition patterns. Chem. Sci. 2020, 11, 384–388.
- 3 Yan, X.-X.; Li, B.; Lin, H.-S.; Jin, F.; Niu, C.; Liu, K.-Q.; Wang, G.-W.; Yang, S. Successively Regioselective Electrosynthesis and Electron Transport Property of Stable Multiply Functionalized [60]Fullerene Derivatives. Research 2020, 2020, 2059190.
- 4 Yan, X.-X.; Niu, C.; Yin, Z.-C.; Lu, W.-Q.; Wang, G.-W. Anionic alkene-azide cycloaddition (AAAC) strategy toward electrosynthesis of multifunctionalized [60]fullerene derivatives and further applications. Sci. Bull. 2022, 67, 2406–2410.
- 5 Cardullo, F.; Seiler, P.; Isaacs, L.; Nierengarten, J. F.; Haldimann, R. F.; Diederich, F.; Mordasini-Denti, T.; Thiel, W.; Boudon, C.; Gisselbrecht, J. P.; Gross, M. Bis- through Tetrakis-Adducts of C60 by Reversible Tether-Directed Remote Functionalization and systematic investigation of the changes in fullerene properties as a function of degree, pattern, and nature of functionalization. Helv. Chim. Acta 1997, 80, 343–371.
- 6 Matsuo, Y.; Nakamura, E. Selective Multiaddition of Organocopper Reagents to Fullerenes. Chem. Rev. 2008, 108, 8, 3016–3028.
- 7 Beil, S. B.; von Delius, M. Supramolecular Approaches for Taming the Chemo- and Regiochemistry of C60 Addition Reactions. Org. Mater. 2021, 3, 146–154.
- 8 Khakina, E. A.; Troshin, P. A. Halogenated fullerenes as precursors for the synthesis of functional derivatives of C60 and C70. Russ. Chem. Rev. 2017, 86, 805–830.
- 9 Bianco, A.; Da Ros, T., Prato, M.; Toniolo, C. Fullerene-based Amino Acids and Peptides. J. Peptide Sci. 2001, 7, 208–219.
- 10 Lin, M.-S.; Chen, R.-T.; Yu, N.-Y.; Sun, L.-C.; Liu, Y.; Cui, C.-H.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Fullerene-based amino acid ester chlorides self-assembled as spherical nano-vesicles for drug delayed release. Colloids Surf. B Biointerfaces 2017, 159, 613–619.
- 11 Hsieh, F.-Y.; Zhilenkov, A. V.; Voronov, I. I.; Khakina, E. A.; Mischenko, D. V.; Troshin, P. A.; Hsu, S.-h. Water-Soluble Fullerene Derivatives as Brain Medicine: Surface Chemistry Determines If They Are Neuroprotective and Antitumor. ACS Appl. Mater. Interfaces 2017, 9, 11482−11492.
- 12 Yang, J.; Alemany, L. B.; Driver, J.; Hartgerink, J. D.; Barron, A. R. Fullerene-Derivatized Amino Acids: Synthesis, Characterization, Antioxidant Properties, and Solid-Phase Peptide Synthesis. Chem. - Eur. J. 2007, 13, 2530–2545.
- 13 Pochkaeva, E. I.; Podolsky, N. E.; Zakusilo, D. N.; Petrov, A. V.; Charykov, N. A.; Vlasov, T. D.; Penkova, A. V.; Vasina, L. V.; Murin, I. V.; Sharoyko, V. V.; Semenov, K. N. Fullerene derivatives with amino acids, peptides and proteins: From synthesis to biomedical application. Prog. Solid. State Chem. 2020, 57, 100255.
- 14 Kornev, A. B.; Khakina, E. A.; Troyanov, S. I.; Kushch, A. A.; Peregudov, A.; Vasilchenko, A.; Deryabin, D. G.; Martynenko, V. M.; Troshin, P. A. Facile preparation of amine and amino acid adducts of [60]fullerene using chlorofullerene C60Cl6 as a precursor. Chem. Commun. 2012, 48, 5461–5463.
- 15 Bianco, A.; Maggini, M.; Scorrano, G.; Toniolo, C.; Marconi, G.; Villani, C.; Prato, M. Synthesis, Chiroptical Properties, and Configurational Assignment of Fulleroproline Derivatives and Peptides J. Am. Chem. Soc. 1996, 118, 4072–4080.
- 16 Pellarini, F.; Pantarotto, D.; Da Ros, T.; Giangaspero, A.; Tossi, A.; Prato, M. A Novel [60]Fullerene Amino Acid for Use in Solid-Phase Peptide Synthesis. Org. Lett. 2001, 3, 1845–1848.
- 17 Ravanello, B. B.; Seixas, N.; Rodrigues, O. E. D.; da Silva, R. S.; Villetti, M. A.; Frolov, A.; Rivera, D. G.; Westermann, B. Diversity Driven Decoration and Ligation of Fullerene by Ugi and Passerini Multicomponent Reactions. Chem. - Eur. J. 2018, 24, 9788–9793.
- 18 Kraevaya, O. A.; Peregudov, A. S.; Godovikov, I. A.; Shchurik, E. V.; Martynenko, V. M.; Shestakov, A. F.; Balzarini, J.; Schols, D.; Troshin, P. A. Direct arylation of C60Cl6 and C70Cl8 with carboxylic acids: a synthetic avenue to water-soluble fullerene derivatives with promising antiviral activity. Chem. Commun. 2020, 56, 1179–1182.
- 19 Kraevaya, O. A.; Peregudov, A. S.; Troyanov, S. I.; Godovikov, I.; Fedorova, N. E.; Klimova, R. R.; Sergeeva, V. A.; Kameneva, L. V.; Ershova, E. S.; Martynenko, V. M.; Claes, S.; Kushch, A. A.; Kostyuk, S. V.; Schols, D.; Shestakov, A. F.; Troshin, P. A. Diversion of the Arbuzov Reaction: Alkylation of C-Cl Instead of Phosphonic Ester Formation on the Fullerene Cage. Org. Biomol. Chem. 2019, 17, 7155–7160.
- 20 Liu, X.; Jiao, W.; Lei, M.; Zhou, Y.; Song, B.; Li, Y. Crown-ether functionalized fullerene as a solution-processable cathode buffer layer for high performance perovskite and polymer solar cells. J. Mater. Chem. A 2015, 3, 9278–9284.
- 21 Sakai-Otsuka, Y.; Ogawa, Y.; Satoh, T.; Chen, W.-C.; Borsali, R. Carbohydrate-attached fullerene derivative for selective localization in ordered carbohydrate-block-poly(3-hexylthiophene) nanodomains. Carbohydr. Polym. 2021, 255, 117528.
- 22 Zarubaev, V.; Anfimov, P.; Shtro, A.; Rasnetsov, L.; Kiselev, O. Activity of a Novel Fullerene-based Antiviral Against Influenza Virus Infection In Vitro and In Vivo. Antivir. Res. 2010, 86, A50–A51.
- 23 Du, C.-X.; Xiong, H.-R.; Ji, H.; Liu, Q.; Xiao, H.; Yang, Z.-Q. The antiviral effect of fullerene-liposome complex against influenza virus (H1N1) in vivo. Sci. Res. Essays 2012, 7, 706–711.
- 24 Shoji, M.; Takahashi, E.; Hatakeyama, D.; Iwai, Y.; Morita, Y.; Shirayama, R.; Echigo, N.; Kido, H.; Nakamura, S.; Mashino, T.; Okutani, T.; Kuzuhara, T. Anti-Influenza Activity of C60 Fullerene Derivatives. PLoS One 2013, e66337.
- 25 Tollas, S.; Bereczki, I.; Borbás, A.; Batta, G.; Vanderlinden, E.; Naesens, L.; Herczegh, P. Synthesis of a cluster-forming sialylthio-D-galactose fullerene conjugate and evaluation of its interaction with influenza virus hemagglutinin and neuraminidase. Bioorg. Med. Chem. Lett. 2014, 24, 2420–2423.
- 26 Khalikov, S. K.; Sharipova, D.; Zafarov, S. Z.; Umarkhon, M.; Alieva, S. V. Synthesis and Characterization of Fullero-C60 α-Amino Acids with Antiviral Properties. Chem. Nat. Compd. 2017, 53, 121–127.
- 27 Zhu, X.; Xiao, S.; Zhou, D.; Sollogoub, M.; Zhang, Y. Design, synthesis and biological evaluation of water-soluble per-Omethylated cyclodextrin-C60 conjugates as anti-influenza virus agents. Eur. J. Med. Chem. 2018, 146, 194–205.
- 28 Kornev, A. B.; Peregudov, A. S.; Martynenko, V. M.; Balzarini, J.; Hoorelbeke, B.; Troshin, P. A. Synthesis and antiviral activity of highly water-soluble polycarboxylic derivatives of [70]fullerene. Chem. Commun. 2011, 47, 8298–8300.
- 29 Sinegubova, E. O.; Kraevaya, O. A.; Volobueva, A. S.; Zhilenkov, A. V.; Shestakov, A. F.; Baykov, S. V.; Troshin, P. A.; Zarubaev, V. V. Water-Soluble Fullerene C60 Derivatives are Effective Inhibitors of Influenza Virus Reproduction. Microorganisms 2023, 11, 681.
- 30 Kraevaya, O. A.; Bolshakova, V. S.; Peregudov, A. S.; Chernyak, A. V.; Slesarenko, N. A.; Markov, V. Y.; Lukonina, N. S.; Martynenko, V. M.; Sinegubova, E. O.; Shestakov, A. F.; Zarubaev, V. V.; Schols, D.; Troshin, P. A. Water-Promoted Reaction of C60Ar5Cl Compounds with Thiophenes Delivers a Family of Multifunctional Fullerene Derivatives with Selective Antiviral Properties. Org. Lett. 2021, 23, 18, 7226–7230.
1 August, 2023
Pages 1803-1808




