Cu(OAc)2-Mediated Synthesis of Fullerodihydropyridine-3-ones via the Reaction of [60]Fullerene with β-Substituted Ethylamines in the Absence or Presence of Arylacetaldehydes†
Xiu-Shan Liu
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
‡ These authors contributed equally to this work.
† Dedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Search for more papers by this authorHui-Juan Wang
State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Center for Magnetic Resonance, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, Wuhan, Hubei, 430071 China
‡ These authors contributed equally to this work.
† Dedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Search for more papers by this authorFei-Lun Wu
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
‡ These authors contributed equally to this work.
† Dedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Search for more papers by this authorJing-Wen Huo
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
Search for more papers by this authorXing-Yu Wang
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
Search for more papers by this authorCorresponding Author
Fa-Bao Li
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
Collaborative Innovation Center for Advanced Organic Chemical Materials Co-constructed by the Province and Ministry, Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules, College of Chemistry and Chemical Engineering, Hubei University, Wuhan, Hubei, 430062 China
E-mail: [email protected]; [email protected]Search for more papers by this authorRui Sun
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
Search for more papers by this authorLi Liu
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
Search for more papers by this authorCorresponding Author
Chao-Yang Liu
State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Center for Magnetic Resonance, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, Wuhan, Hubei, 430071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorXiu-Shan Liu
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
‡ These authors contributed equally to this work.
† Dedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Search for more papers by this authorHui-Juan Wang
State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Center for Magnetic Resonance, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, Wuhan, Hubei, 430071 China
‡ These authors contributed equally to this work.
† Dedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Search for more papers by this authorFei-Lun Wu
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
‡ These authors contributed equally to this work.
† Dedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Search for more papers by this authorJing-Wen Huo
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
Search for more papers by this authorXing-Yu Wang
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
Search for more papers by this authorCorresponding Author
Fa-Bao Li
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
Collaborative Innovation Center for Advanced Organic Chemical Materials Co-constructed by the Province and Ministry, Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules, College of Chemistry and Chemical Engineering, Hubei University, Wuhan, Hubei, 430062 China
E-mail: [email protected]; [email protected]Search for more papers by this authorRui Sun
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
Search for more papers by this authorLi Liu
State Key Laboratory of Biocatalysis and Enzyme Engineering, School of Life Sciences, Hubei University, Wuhan, Hubei, 430062 China
Search for more papers by this authorCorresponding Author
Chao-Yang Liu
State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Center for Magnetic Resonance, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences, Wuhan, Hubei, 430071 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
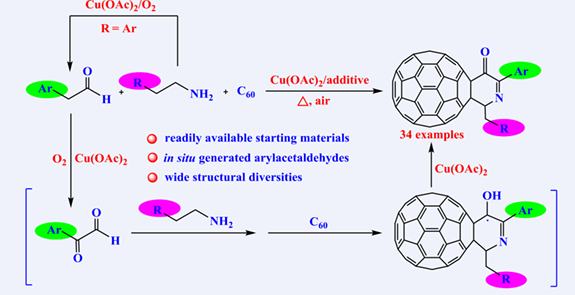
A series of unreported fullerodihydropyridine-3-ones were synthesized as a new family of fullerene derivatives in moderate to good yields by a simple one-step reaction of [60]fullerene with cheap and readily available β-substituted ethylamines in the absence or presence of arylacetaldehydes under the assistance of Cu(OAc)2. The in situ generation of arylacetaldehydes by the C—N bond cleavage of arylethylamines avoided their complex synthesis in advance and realized the preparation of fullerodihydropyridine-3-ones with structural and functional diversities, which may have promising applications in perovskite solar cells to improve the performance of photovoltaic devices due to the existence of a large π-conjugated system on the dihydropyridine-3-one ring.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200717-sup-0001-Supinfo.pdfPDF document, 15.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see: (a) Hirsch, A. Addition Reactions of Buckminsterfullerene (C60). Synthesis 1995, 8, 895–913;
10.1055/s-1995-4046 Google Scholar(b) Yurovskaya, M. A.; Trushkov, I. V. Cycloaddition to Buckminsterfullerene C60: Advancements and Future Prospects. Russ. Chem. Bull., Int. Ed. 2002, 51, 367–443; (c) Matsuo, Y.; Nakamura, E. Selective Multiaddition of Organocopper Reagents to Fullerenes. Chem. Rev. 2008, 108, 3016–3028; (d) Tzirakis, M. D.; Orfanopoulos, M. Radical Reactions of Fullerenes: from Synthetic Organic Chemistry to Materials Science and Biology. Chem. Rev. 2013, 113, 5262–5321; (e) Zhu, S.-E; Li, F.; Wang, G.-W. Mechanochemistry of Fullerenes and Related Materials. Chem. Soc. Rev. 2013, 42, 7535–7570; (f) Maroto, E. E.; Izquierdo, M.; Reboredo, S.; Marco-Martínez, J.; Filippone, S.; Martín, N. Chiral Fullerenes from Asymmetric Catalysis. Acc. Chem. Res. 2014, 47, 2660–2670; (g) Wang, G.-W. Fullerene Mechanochemistry: Serendipitous Discovery of Dumb-Bell-Shaped C120 and Beyond. Chin. J. Chem. 2021, 39, 1797–1803. For selected papers, see: (h) Zhou, Z.; Han, H.; Chen, Z.; Gao, R.; Liu, Z.; Su, J.; Xin, N.; Yang, X.; Gan, L.-B. Concise Synthesis of Open-Cage Fullerenes for Oxygen Delivery. Angew. Chem. Int. Ed. 2019, 58, 17690–17694; (i) Niu, C.; Liu, Z.; Chen, M.; Yang, S.; Wang, G.-W. Unexpected Formation of Pyrazoline-Fused Metallofullerenes from the Multicomponent Cascade Reaction of Sc3N@Ih-C80 with Tetrazines, Water, and Oxygen. Org. Lett. 2022, 24, 3493–3498; (j) Liu, T.-X.; Zhu, X.; Xia, S.; Wang, X.; Zhang, P.; Zhang, G. NHC-Catalyzed Three-Component Hydroalkylation Reactions of [60]Fullerene: An Umpolung Approach to Diverse Monoalkylated Hydrofullerenes. Org. Lett. 2022, 24, 3691–3695; (k) Su, Y.-T.; Yin, Z.-C.; Wang, G.-W. Palladium-Catalyzed Three-Component 1,4-Aminoarylation of [60]Fullerene with Aryl Iodides and N-Methoxysulfonamides, and Further Transformations. Org. Chem. Front. 2022, 9, 2739–2745; (l) Yin, Z.-C.; Niu, C.; Li, M.; Liu, W.-R.; Wang, G.-W. Regioselective Electrochemical Hydroalkylations of [60]Fullerene-Fused Furochromenone. Chin. J. Chem. 2023, 41, 769–775.
- 2(a) Wang, H.; Cai, F.; Zhang, M.; Wang, P.; Yao, J.; Gurney, R. S.; Li, F.; Liu, D.; Wang, T. Halogen-Substituted Fullerene Derivatives for Interface Engineering of Perovskite Solar Cells. J. Mater. Chem. A 2018, 6, 21368–21378; (b) Wang, H.; Li, F.; Wang, P.; Sun, R.; Ma, W.; Chen, M.; Miao, W.; Liu, D.; Wang, T. Chlorinated Fullerene Dimers for Interfacial Engineering Toward Stable Planar Perovskite Solar Cells with 22.3% Efficiency. Adv. Energy Mater. 2020, 10, 2000615; (c) Wang, H.; Chen, M.; Li, F.; Sun, R.; Wang, P.; Ye, F.; Zhang, H.; Miao, W.; Liu, D.; Wang, T. Thiophene Terminated Fullerene Derivatives for Interfacial Modification toward High Efficiency MAPbI3 Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 9824–9832; (d) Yan, X.-X.; Li, B.; Lin, H.-S.; Jin, F.; Niu, C.; Liu, K.-Q.; Wang, G.-W.; Yang, S. Successively Regioselective Electrosynthesis and Electron Transport Property of Stable Multiply Functionalized [60]Fullerene Derivatives. Research 2020, 2020, 2059190; (e) Liu, Z.; Yin, Z.-C.; Lu, W.-Q.; Niu, C.; Chen, M.; Yang, S.; Wang, G.-W. Cu(I)-Catalyzed Synthesis of [60]Fullerene-Fused Lactams and Further Electrochemical Functionalization. Org. Lett. 2021, 23, 4051–4056; (f) Yan, X.-X.; Niu, C.; Yin, Z.-C.; Lu, W.-Q.; Wang, G.-W. Anionic Alkene-Azide Cycloaddition (AAAC) Strategy toward Electrosynthesis of Multifunctionalized [60]Fullerene Derivatives and Further Applications. Sci. Bull. 2022, 67, 2406–2410.
- 3(a) Shi, J.-L.; Li, F.-B.; Zhang, X.-F.; Wu, J.; Zhang, H.-Y.; Peng, J.; Liu, C.-X.; Liu, L.; Wu, P.; Li, J.-X. Synthesis and Functionalization of Symmetrical 2,5-Diaryl Fulleropyrrolidines: Ferric Perchlorate-Mediated One-Step Reaction of [60]Fullerene with Arylmethanamines. J. Org. Chem. 2016, 81, 1769–1777; (b) Shi, J.-L.; Zhang, X.-F.; Wang, H.-J.; Li, F.-B.; Zhong, X.-X.; Liu, C.-X.; Liu, L.; Liu, C.-Y.; Qin, H.-M.; Huang, Y.-S. A Protocol for the Preparation of 2,5-Diaryl Fulleropyrrolidines: Thermal Reaction of [60]Fullerene with Aromatic Aldehydes and Arylmethanamines. J. Org. Chem. 2016, 81, 7662–7674; (c) Zhang, M.; Wang, H.-J.; Li, F.-B.; Zhong, X.-X.; Huang, Y.-S.; Liu, L.; Liu, C.-Y.; Asiri, A. M.; Alamry, K. A. Synthesis of 2-Aryl-5-alkyl-fulleropyrrolidines: Metal-Free-Mediated Reaction of [60]Fullerene with Aromatic Aldehydes and Inactive Primary Amines. J. Org. Chem. 2017, 82, 8617–8627; (d) Huang, C.; Huang, G.; Wang, H.-J.; Li, F.-B.; Wang, Z.; Huang, Y.; Liu, L.; Liu, C.-Y. N-Alkylation of Fulleropyrrolidines by Aminomethylation Reaction of Ketones/Arylboronic Acids. Adv. Synth. Catal. 2018, 360, 3732–3750; (e) Li, Y.-F.; Zhang, D.; Wang, H.-J.; Li, F.-B.; Sun, L.; Liu, L.; Liu, C.-Y.; Asiri, A. M.; Alamry, K. A. Metal-Free Synthesis of N-Alkyl-2,5-Unsubstituted/Monosubstituted Fulleropyrrolidines: Reaction of [60]Fullerene with Paraformaldehyde and Amines. J. Org. Chem. 2019, 84, 2922–2832; (f) Niu, C.; Chen, X.-P.; Yin, Z.-C.; Wang, G.-W. Multicomponent Synthesis of 2-Arylvinyl-Substituted Fulleropyrrolidines from [60]Fullerene, Amines and Aldehydes. Eur. J. Org. Chem. 2019, 2019, 6504–6509.
- 4(a) Wu, J.; Liu, C.–X.; Wang, H.-J.; Li, F.-B.; Shi, J.-L.; Liu, L.; Li, J.-X.; Liu, C.-Y.; Huang, Y.-S. Cu(OAc)2-Mediated Reaction of [60]Fullerene with Aldehydes and Primary Amines for the Synthesis of Fulleropyrrolines. J. Org. Chem. 2016, 81, 9296–9307; (b) Peng, J.; Xiang, J.-J.; Wang, H.-J.; Li, F.-B.; Huang, Y.-S.; Liu, L.; Liu, C.-Y.; Asiri, A. M.; Alamry, K. A. DMAP-Mediated Synthesis of Fulleropyrrolines: Reaction of [60]Fullerene with Aromatic Aldehydes and Arylmethanamines in the Absence or Presence of Manganese(III) Acetate. J. Org. Chem. 2017, 82, 9751–9764; (c) Xiang, J.-J.; Huang, C.; Wang, H.-J.; Li, F.-B.; Shi, J.-L.; Huang, Y.-S.; Liu, L.; Liu, C.-Y.; Asiri, A. M.; Alamry, K. A. Metal-Free-Mediated Synthesis of Fulleropyrrolines by the Reaction of [60]Fullerene with β-Substituted Ethylamines. New J. Chem. 2017, 41, 8725–8728.
- 5 Huang, G.; Zhang, M.; Wang, H.-J.; Li, F.-B.; Yang, F.; Liu, L.; Liu, C.-Y.; Asiri, A. M.; Alamry, K. A. Metal-Free Synthesis of Fulleropyrrolidin- 2-ols: a Novel Reaction of [60]Fullerene with Amines and 2,2-Disubstituted Acetaldehydes. Org. Biomol. Chem. 2018, 16, 7648–7656.
- 6 Peng, J.; Huang, G.; Wang, H.-J.; Li, F.-B.; Huang, C.; Xiang, J.-J.; Huang, Y.; Liu, L.; Liu, C.-Y.; Asiri, A. M.; Alamry, K. A. TEMPO- Mediated Synthesis of Tetrahydropyridinofullerenes: Reaction of [60]Fullerene with α-Methyl-Substituted Arylmethanamines and Aldehydes in the Presence of 4-Dimethylaminopyridine. J. Org. Chem. 2018, 83, 85–95.
- 7 Liu, X.; Wang, X.-Y.; Sun, R.; Huang, M.-R.; Liu, X.-S.; Wang, H.-J.; Li, F.-B.; Liu, X.-F.; Liu, L.; Liu, C.-Y. Fullerotetrahydroquinolines: TfOH/ TsOH·H2O-Mediated One-Pot Two-Step Synthesis and N-Alkylation/ Acylation/Carboamidation Reaction. Adv. Synth. Catal. 2021, 363, 4399–4421.
- 8(a) Ma, W.; Wang, K.; Huang, C.; Wang, H.–J.; Li, F.-B.; Sun, R.; Liu, L.; Liu, C.-Y.; Asiri, A. M. Stereoselective Synthesis of Amino-Substituted Cyclopentafullerenes Promoted by Magnesium Perchlorate/Ferric Perchlorate. Org. Biomol. Chem. 2020, 18, 964–974; (b) Li, Y.-F.; Wang, K.; Wang, H.-J.; Li, F.-B.; Sun, R.; Li, J.-X.; Liu, L.; Liu, C.-Y.; Asiri, A. M. Facile Access to Amino-Substituted Cyclopentafullerenes: Novel Reaction of [60]Fullerene with β-Substituted Propionaldehydes and Secondary Amines in the Absence/Presence of Magnesium Perchlorate. Org. Biomol. Chem. 2020, 18, 6866–6880; (c) Niu, C.; Chen, X.-P.; Yin, Z.-C.; Wang, W.-F.; Wang, G.-W. Alternative Access to Cyclopentafullerenes through the Reaction of [60]Fullerene with Aldehydes and Secondary Amines. J. Org. Chem. 2020, 85, 6878–6887.
- 9 Lu, X.-W.; Xing, M.-L.; Miao, C.-B.; Li, J.-X.; Sun, X.-Q.; Yang, H.-T. Cu(OAc)2-Promoted Reaction of [60]Fullerene with Primary Amines or Diamines. Org. Biomol. Chem. 2015, 13, 8405–8410.
- 10(a) Stojiljković, A.; Andrejević, V.; Mihailovi, M. Lj. The Reaction of Lead Tetraacetate with Primary and Secondary Amines Containing an α-Methylene Group. Tetrahedron 1967, 23, 721–732; (b) Orito, K.; Hatakeyama, T.; Takeo, M.; Uchiito, S.; Tokuda, M.; Suginome, H. Dimerization of Anilines and Benzylamines with Mercury(II) Oxide-Iodine Reagent. Tetrahedron 1998, 54, 8403–8410.
- 11 Zhang, L.; Ang, G. Y.; Chiba, S. Copper-Catalyzed Benzylic C-H Oxygenation under an Oxygen Atmosphere via N-H Imines as an Intramolecular Directing Group. Org. Lett. 2011, 13, 1622–1625.
- 12 Guo, S.; Hua, J.; Dai, Z.; Yang, Z.; Fang, Z.; Guo, K. Two-Step Continuous Synthesis of Dicarbonyl Indoles via I2/DMSO-Promoted Oxidative Coupling: A Green and Practical Approach to Valuable Diketones from Aryl Acetaldehydes. ACS Sustainable Chem. Eng. 2018, 6, 7979–7988.
- 13 Kasséhin, U. C.; Gbaguidi, F. A.; McCurdy, C. R.; Poupaert, J. H. Synthesis of Antitrypanosomal Thiosemicarbazones Using Anthranilic Acid as an Innovative Green Nucleophilic Catalyst. J. Chem. Pharm. Res. 2014, 6, 607–612.




