Stereoselective Synthesis of Nontethered trans-4 Bis(aziridino)[60]fullerene Derivatives†
Jie Xiong
State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology, Mianyang, Sichuan, 621010 China
These authors contributed equally to this work and should be considered co-first authors.
Search for more papers by this authorShuang Feng
State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology, Mianyang, Sichuan, 621010 China
These authors contributed equally to this work and should be considered co-first authors.
Search for more papers by this authorRufang Peng
State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology, Mianyang, Sichuan, 621010 China
Search for more papers by this authorCorresponding Author
Bo Jin
State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology, Mianyang, Sichuan, 621010 China
E-mail: [email protected]Search for more papers by this authorJie Xiong
State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology, Mianyang, Sichuan, 621010 China
These authors contributed equally to this work and should be considered co-first authors.
Search for more papers by this authorShuang Feng
State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology, Mianyang, Sichuan, 621010 China
These authors contributed equally to this work and should be considered co-first authors.
Search for more papers by this authorRufang Peng
State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology, Mianyang, Sichuan, 621010 China
Search for more papers by this authorCorresponding Author
Bo Jin
State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology, Mianyang, Sichuan, 621010 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of Recent Advances in Fullerene Chemistry.
Comprehensive Summary
The stereoselective preparation of fullerene bis-adducts through nontethered methods remains difficult due to the significant amount of regioisomers produced. The trans-4 aziridino[60]fullerenes, C60(NC6H4R)n (n = 1 or 2, R = OMe, OEt, OBu), were selectively synthesized even without a catalyst by reacting octabromofullerene with the corresponding aniline. Nuclear magnetic resonance spectroscopy, UV-vis spectroscopy, and X-ray structural analysis provided convincing characterization of the compounds. A possible reaction process was proposed to clarify the synthesis of highly regioselective trans-4-bisaziridino[60]fullerenes. The possible application of these aziridino[60]fullerene derivatives as propellant stabilizers was also explored.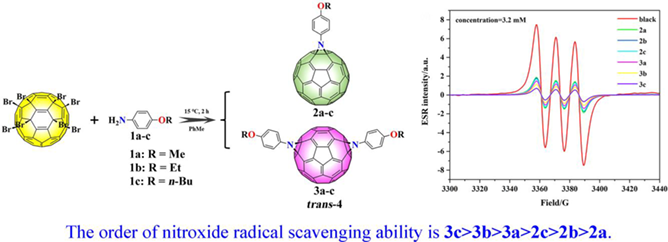
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300094-sup-0001-supinfo.pdfPDF document, 9.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Hirsch, A. Fullerenes and Related Structures. Top. Curr. Chem. 1999, 199, 173–187.
- 2 Nakamura, E.; Isobe, H. Functionalized Fullerenes in Water. The First 10 Years of Their Chemistry, Biology, and Nanoscience. Acc. Chem. Res. 2003, 36, 807–815.
- 3 Guldi, D. M.; Zerbetto, F.; Georgakilas, V.; Prato, M. Ordering Fullerene Materials at Nanometer Dimensions. Acc. Chem. Res. 2005, 38, 38–43.
- 4 Li, C. Z.; Yip, H. L.; Jen, A. K. Y. Functional fullerenes for organic photovoltaics. J. Mater. Chem. 2012, 22, 4161–4177.
- 5 Kirner, S.; Sekita, M.; Guldi, D. M. 25th Anniversary Article: 25 Years of Fullerene Research in Electron Transfer Chemistry. Adv. Mater. 2014, 26, 1482–1493.
- 6 Taylor, R.; Wasserman, E. The pattern of additions to fullerenes. Philos. Trans. Royal Soc. A 1993, 343, 87–101.
- 7 Bent, H. A. An Appraisal of Valence-bond Structures and Hybridization in Compounds of the First-row Elements. Chem. Rev. 1961, 61, 275–311.
- 8 Hirsch, A.; Brettreich, M. Fullerenes: Chemistry and Reactions, John Wiley & Sons, 2006.
- 9
Langa, F.; Nierengarten, J. F. Fullerenes: Principles and Applications, Royal Society of Chemistry, 2011.
10.1039/9781849732956 Google Scholar
- 10 Fakharuddin, A.; Armadorou, K. K.; Zorba, L. P.; Tountas, M.; Seewald, T.; Soultati, A.; Tsipas, P.; Schütz, E. R.; Tzoganakis, N.; Panagiotakis, S.; Yannakopoulou, K.; Dimoulas, A.; Psycharis, V.; Kymakis, E.; Yusoff, A.; Aidinis, K.; Schmidt-Mende, L.; Vougioukalakis, G. C.; Nazeeruddin, M. K.; Vasilopoulou, M. A triethyleneglycol C60 mono-adduct derivative for efficient electron transport in inverted perovskite solar cells. Chin. J. Chem. 2022, 41, 431–442.
- 11 Barham, J. P.; Tanaka, S.; Koyama, E.; Ohneda, N.; Okamoto, T.; Odajima, H.; Sugiyama, J.; Norikane, Y. Selective, Scalable Synthesis of C60-Fullerene/Indene Monoadducts Using a Microwave Flow Applicator. J. Org. Chem. 2018, 83, 4348–4354.
- 12 Li, G.; Jin, B.; Chai, Z. H.; Ding, L.; Chu, S. J.; Peng, R. F. Synthesis and crystal characterization of novel fulleropyrrolidines and their potential application as nitrocellulose-based propellants stabilizer. Polym. Degrad. Stabil. 2020, 172, 109061.
- 13 Zheng, T.; Shan, D. S.; Jin, B.; Peng, R. F. Synthesis and self-sensitized photo-oxidation of 2-fulleropyrrolines by palladium(ii)-catalyzed heteroannulation of [60]fullerene with benzoyl hydrazone esters. Org. Biomol. Chem. 2018, 16, 8845–8853.
- 14 Zhang, S.; Li, M.; Zeng, H.; Zheng, X.; Luo, L.; You, S.; Zhao, Y.; Liu, R.; Tian, C.; Li, X. Grain Boundary and Buried Interface Suturing Enabled by Fullerene Derivatives for High-Performance Perovskite Solar Module. ACS Energy Lett. 2022, 7, 3958–3966.
- 15 Zhou, W.; Jia, L.; Chen, M.; Li, X.; Su, Z.; Shang, Y.; Jiang, X.; Gao, X.; Chen, T.; Wang, M.; Zhu, Z.; Lu, Y.; Yang, S. An Improbable Amino-Functionalized Fullerene Spacer Enables 2D/3D Hybrid Perovskite with Enhanced Electron Transport in Solar Cells. Adv. Funct. Mater. 2022, 32, 2201374.
- 16 Cerón, M. R.; Izquierdo, M.; Aghabali, A.; Vogel, S. P.; Olmstead, M. M.; Balch, A. L.; Echegoyen, L. Tethered bis-pyrrolidine additions to C70: Some unexpected and new regioisomers. Carbon 2016, 105, 394–400.
- 17 Nakamura, Y.; Takano, N.; Nishimura, T.; Yashima, E.; Sato, M.; Kudo, T.; Nishimura, J. First Isolation and Characterization of Eight Regioisomers for [60]Fullerene−Benzyne Bisadducts. Org. Lett. 2001, 3, 1193–1196.
- 18 Cerón, M. R.; Echegoyen, L. Recent progress in the synthesis of regio-isomerically pure bis-adducts of empty and endohedral fullerenes. J. Phys. Org. Chem. 2016, 29, 613–619.
- 19 Biglova, Y. N.; Kraikin, V. A.; Torosyan, S. A.; Mikheev, V. V.; Mustafin, A. G.; Kolesov, S. V.; Miftakhov, M. S. UV spectroscopy of methanofullerene derivatives with different degrees of substitution. Russ. J. Phys. Chem. A 2013, 87, 1692–1695.
- 20 Isaacs, L.; Haldimann, R. F.; Diederich, F. Tether-Directed Remote Functionalization of Buckminsterfullerene: Regiospecific Hexaadduct Formation. Angew. Chem. Int. Ed. Engl. 1994, 33, 2339–2342.
- 21 Ishi-I, T.; Shinkai, S. Regioselective introduction of two boronic acid groups into [60]fullerene using saccharides as imprinting templates. Chem. Commun. 1998, 9, 1047–1048.
- 22 Izquierdo, M.; Cerón, M. R.; Olmstead, M. M.; Balch, A. L.; Echegoyen, L. [5,6]-Open Methanofullerene Derivatives of Ih-C80. Angew. Chem. Int. Ed. 2013, 52, 11826–11830.
- 23
Bourgeois, J. P.; Echegoyen, L.; Fibbioli, M.; Pretsch, E.; Diederich, F. Regioselective Synthesis of trans-1 Fullerene Bis-Adducts Directed by a Crown Ether Tether: Alkali Metal Cation Modulated Redox Properties of Fullerene-Crown Ether Conjugates. Angew. Chem. Int. Ed. 1998, 37, 2118–2121.
10.1002/(SICI)1521-3773(19980817)37:15<2118::AID-ANIE2118>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 24 Ueno, H.; Uchiyama, K.; Ma, Y.; Watanabe, K.; Yoza, K.; Matsuo, Y.; Moriyama, H. Octaalkoxyfullerenes: Widely LUMO-Tunable C2v-Symmetric Fullerene Derivatives. J. Org. Chem. 2018, 83, 10655–10659.
- 25 Khakina, E. A.; Kraevaya, O. A.; Popova, M. L.; Peregudov, A. S.; Troyanov, S. I.; Chernyak, A. V.; Martynenko, V. M.; Kulikov, A. V.; Schols, D.; Troshin, P. A. Synthesis of different types of alkoxy fullerene derivatives from chlorofullerene C60Cl6. Org. Biomol. Chem. 2017, 15, 773–777.
- 26 Kraevaya, O. A.; Peregudov, A. S.; Godovikov, I. A.; Shchurik, E. V.; Martynenko, V. M.; Shestakov, A. F.; Balzarini, J.; Schols, D.; Troshin, P. A. Direct arylation of C60Cl6 and C70Cl8 with carboxylic acids: a synthetic avenue to water-soluble fullerene derivatives with promising antiviral activity. Chem. Commun. 2020, 56, 1179–1182.
- 27 Zhao, Y.; Jin, B.; Ding, L.; Xiao, L. P. C.; Peng, R. F. Regioselective synthesis of 4,11,15,30-tetraalkoxyphenyl fullereno[1,2:2’,3’]dihydrobenzofurans and potential application as propellant stabilizer. Tetrahedron Lett. 2020, 61, 152009–152013.
- 28 Lou, N.; Kraevaya, O. A.; Troshin, P. A.; Gan, L. B. Synthesis of Pentapyrazolyl, Pentapyrrolyl, and Pentaanilino C60 Derivatives. Synthesis 2018, 50, 4283–4289.
- 29 Ding, L.; Jin, B.; Guo, Z. C.; Zhao, Y.; Chen, J. J.; Peng, R. F. Regioselective Synthesis and Crystallographic Characterization of Nontethered cis-1 and cis-2 Bis(benzofuro)[60]fullerene Derivatives. Org. Lett. 2019, 21, 9924–9928.
- 30 Khalilov, M.; Tulyabaev, A. R.; Akhmetov, A. R.; Tuktarov, A. R. Synthesis and 13C NMR features of N-substituted aziridino[60]fullerenes. Russ. Chem. Bull. 2015, 64, 2725–2730.
- 31 Ito, H.; Ishida, Y.; Saigo, K. Preparation and identification of bis(formylmethano)[60]fullerene isomers: the first systematic study on bifunctionalized [60]fullerenes with dissymmetric addends. Tetrahedron Lett. 2005, 46, 8757–8760.
- 32 Yang, H. T.; Liang, X. C.; Wang, Y. H.; Yang, Y.; Sun, X. Q.; Miao, C. B. CuCl2-Mediated Reaction of [60]Fullerene with Amines in the Presence or Absence of Dimethyl Acetylenedicarboxylate: Preparation of Fulleropyrroline or Aziridinofullerene Derivatives. J. Org. Chem. 2013, 78, 11992–11998.
- 33 Averdung, J.; Mattay, J. Exohedral functionalization of [60]fullerene by [3+2] cycloadditions: Syntheses and chemical properties of triazolino-[60]fullerenes and 1,2-(3,4-dihydro-2H-pyrrolo)-[60]fullerenes. Tetrahedron 1996, 52, 5407–5420.
- 34 Tsuruoka, R.; Nagamachi, T.; Murakami, Y.; Komatsu, M.; Minakata, S. Aziridination of C60 with Simple Amides and Catalytic Rearrangement of the Aziridinofullerenes to Azafulleroids. J. Org. Chem. 2009, 74, 1691–1697.
- 35 Bosi, S.; Feruglio, L.; Milic, D.; Prato, M. Synthesis and water solubility of novel fullerene bisadduct derivatives. Eur. J. Org. Chem. 2003, 2003, 4741–4747.
- 36 Isaacs, L.; Haldimann, R. F.; Diederich, F. Tether-Directed Remote Functionalization of Buckminsterfullerene: Regiospecific Hexaadduct Formation. Angew. Chem. Int. Ed. 1994, 33, 2339–2342.
- 37 Rotas, G.; Tagmatarchis, N. Regioselective triphenylamine-tether- directed synthesis of [60]fullerene bis-adducts. Tetrahedron Lett. 2009, 50, 398–401.
- 38 Okada, M.; Nakahodo, T.; Ishitsuka, M. O.; Nikawa, H.; Tsuchiya, T.; Akasaka, T.; Fujie, T.; Yoshimura, T.; Slanina, Z.; Nagase, S. Highly Regioselective Synthesis of Bis-Aziridino[60]fullerene with Sulfilimine. Chem. Asian J. 2011, 6, 416–423.
- 39 Djojo, F.; Herzog, A.; Lamparth, I.; Hampel, F.; Hirsch, A. Regiochemistry of Twofold Additions to [6,6] Bonds in C60: Influence of the Addend-Independent Cage Distortion in 1,2-Monoadducts. Chem. – Eur. J. 1996, 2, 1537–1547.
- 40 Schick, G.; Hirsch, A.; Mauser, H.; Clark, T. Opening and Closure of the Fullerene Cage in cis-Bisimino Adducts of C60: The Influence of the Addition Pattern and the Addend. Chem. – Eur. J. 1996, 2, 935–943.
- 41 Zhao, F.; Meng, X.; Feng, Y.; Jin, Z.; Zhou, Q.; Li, H.; Jiang, L.; Wang, J.; Li, Y.; Wang, C. Single crystalline indene-C60 bisadduct: isolation and application in polymer solar cells. J. Mater. Chem. A 2015, 3, 14991–14995.
- 42 Tao, R.; Umeyama, T.; Kurotobi, K.; Imahori, H. Effects of Alkyl Chain Length and Substituent Pattern of Fullerene Bis-Adducts on Film Structures and Photovoltaic Properties of Bulk Heterojunction Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 17313–17322.
- 43 Trache, D.; Tarchoun, A. F. Stabilizers for nitrate ester-based energetic materials and their mechanism of action: a state-of-the-art review. J. Mater. Sci. 2018, 53, 100–123.
Citing Literature
15 September, 2023
Pages 2282-2288




