Access to Enantioenriched Allylic Alcohols via Peptide-Mimic Phosphonium Salt-Catalyzed Asymmetric Aerobic Hydroxylation
Jixing Che
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
These authors contributed equally.
Search for more papers by this authorSiqiang Fang
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Department of Chemistry, National University of Singapore, Science Drive 3, Singapore, 117543 Singapore
These authors contributed equally.
Search for more papers by this authorZanjiao Liu
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorJiajia He
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorJia-Yan Zheng
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorFan Wang
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorCorresponding Author
Tianli Wang
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
E-mail: [email protected]Search for more papers by this authorJixing Che
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
These authors contributed equally.
Search for more papers by this authorSiqiang Fang
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Department of Chemistry, National University of Singapore, Science Drive 3, Singapore, 117543 Singapore
These authors contributed equally.
Search for more papers by this authorZanjiao Liu
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorJiajia He
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorJia-Yan Zheng
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorFan Wang
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorCorresponding Author
Tianli Wang
Key Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
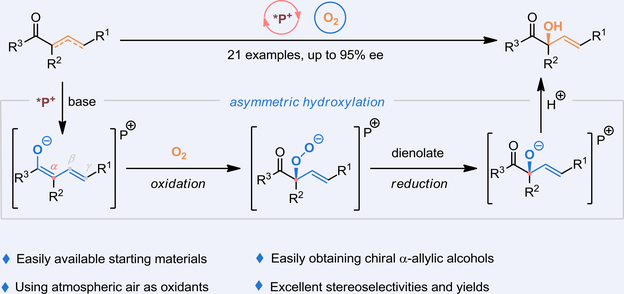
The development of catalytic asymmetric methods that enable access to value-added functionalities or structures, exemplified by allylic alcohols, is a highly interesting yet challenging topic from both academic and industrial perspectives. However, before recent advances in chemical catalysis, there were scarce protocols toward constructing enantioenriched tertiary allylic alcohol scaffolds. In this context, peptide-mimic phosphonium salts were found to be highly efficient in catalytic asymmetric α-hydroxylation of α,β-unsaturated and/or β,ϒ-unsaturated compounds with satisfactory regio- and stereochemical outcomes (up to 97% yield and 95% ee). This methodology tolerates a broad array of substrates and thus provides an expeditious and unified platform for the assembly of enantioenriched tertiary allylic alcohols by avoiding the use of additional reductants and expensive metal catalysts. Furthermore, the power of this protocol is enlarged by simple conditions and the use of air as a source of hydroxyl functionality.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400245-sup-0001-supinfo.pdfPDF document, 13 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Lumbroso, A.; Cooke, M. L.; Breit, B. Catalytic Asymmetric Synthesis of Allylic Alcohols and Derivatives and their Applications in Organic Synthesis. Angew. Chem. Int. Ed. 2013, 52, 1890–1932.
- 2 Hoveyda, A. H.; Evans, D. A.; Fu, G. C. Substrate-directable chemical reactions. Chem. Rev. 1993, 93, 1307–1370.
- 3 Mϋller, P.; Fruit, C. Enantioselective Catalytic Aziridinations and Asymmetric Nitrene Insertions into CH Bonds. Chem. Rev. 2003, 103, 2905–2920.
- 4 Gil, A.; Albericio, F.; Álvarez, M. Role of the Nozaki–Hiyama–Takai–Kishi Reaction in the Synthesis of Natural Products. Chem. Rev. 2017, 117, 8420–8446.
- 5 Gao, J.-M.; Yang, S.-X.; Qin, J.-C. Azaphilones: Chemistry and Biology. Chem. Rev. 2013, 113, 4755–4811.
- 6 Dubost, C.; Markó, I. E.; ́Ryckmans, T. A Concise Total Synthesis of the Lichen Macrolide (+)-Aspicilin. Org. Lett. 2006, 8, 5137–5140.
- 7 Skucas, E.; Ngai, M.-Y.; Komanduri, V.; Krische, M. J. Enantiomerically Enriched Allylic Alcohols and Allylic Amines via C–C Bond-Forming Hydrogenation: Asymmetric Carbonyl and Imine Vinylation. Acc. Chem. Res. 2007, 40, 1394–1401.
- 8 Blakemore, D. C.; Castro, L.; Churcher, I.; Rees, D. C.; Thomas, A. W.; Wilson, D. M.; Wood, A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394.
- 9 Caron, S.; Dugger, R. W.; Ruggeri, S. G.; Ragan, J. A.; Ripin, D. H. B. Large-Scale Oxidations in the Pharmaceutical Industry. Chem. Rev. 2006, 106, 2943–2989.
- 10 Shilov, A. E.; Shteinman, A. A. Oxygen Atom Transfer into C-H Bond in Biological and Model Chemical Systems. Mechanistic Aspects. Acc. Chem. Res. 1999, 32, 763–771.
- 11 Christoffers, J.; Baro, A.; Werner, T. α-Hydroxylation of β-Dicarbonyl Compounds. Adv. Synth. Catal. 2004, 346, 143–151.
- 12 Trost, B. M.; Gnanamani, E.; Kalnmals, C. A.; Hung, C.-I.; Tracy, J. S. Direct Enantio- and Diastereoselective Vinylogous Addition of Butenolides to Chromones Catalyzed by Zn-ProPhenol. J. Am. Chem. Soc. 2019, 141, 1489–1493.
- 13 Zhong, F.; Yue, W.-J.; Zhang, H.-J.; Zhang, C.-Y.; Yin, L. Catalytic Asymmetric Construction of Halogenated Stereogenic Carbon Centers by Direct Vinylogous Mannich-Type Reaction. J. Am. Chem. Soc. 2018, 140, 15170–15175.
- 14 Zhu, B.; Zhang, W.; Lee, R.; Han, Z.; Yang, W.; Tan, D.; Huang, K.; Jiang, Z. Direct Asymmetric Vinylogous Aldol Reaction of Allyl Ketones with Isatins: Divergent Synthesis of 3-Hydroxy-2-Oxindole Derivatives. Angew. Chem. Int. Ed. 2013, 52, 6666–6670.
- 15 Han, B.; He, Z.-Q.; Li, J.-L.; Li, R.; Jiang, K.; Liu, T.-Y.; Chen, Y.-C. Organocatalytic Regio- and Stereoselective Inverse-Electron-Demand Aza-Diels–Alder Reaction of α,β-Unsaturated Aldehydes and N-Tosyl- 1-aza-1,3-butadienes. Angew. Chem. Int. Ed. 2009, 48, 5474–5477.
- 16 Zhang, H.-J.; Schuppe, A. W.; Pan, S.-T.; Chen, J.-X.; Wang, B.-R.; Newhouse, T. R.; Yin, L. Copper-Catalyzed Vinylogous Aerobic Oxidation of Unsaturated Compounds with Air. J. Am. Chem. Soc. 2018, 140, 5300–5310.
- 17 Liang, Y.-F.; Jiao, N. Oxygenation via C–H/C–C Bond Activation with Molecular Oxygen. Acc. Chem. Res. 2017, 50, 1640–1653.
- 18 Tsang, A. S.-K.; Kapat, A.; Schoenebeck, F. Factors That Control C-C Cleavage versus C-H Bond Hydroxylation in Copper-Catalyzed Oxidations of Ketones with O2. J. Am. Chem. Soc. 2016, 138, 518–526.
- 19 Chaudhari, M. B.; Sutar, Y.; Malpathak, S.; Hazra, A.; Gnanaprakasam, B. Transition-Metal-Free C-H Hydroxylation of Carbonyl Compounds. Org. Lett. 2017, 19, 3628–3631.
- 20 Fang, S.; Liu, Z.; Wang, T. Design and Application of Peptide-Mimic Phosphonium Salt Catalysts in Asymmetric Synthesis. Angew. Chem. Int. Ed. 2023, 62, e202307258.
- 21 Wang, H.; Zheng, C.; Zhao, G. Bifunctional Ion Pair Catalysts from Chiral α-Amino Acids. Chin. J. Chem. 2019, 37, 1111–1119.
- 22 Uraguchi, D.; Sakaki, S.; Ooi, T. Chiral Tetraaminophosphonium Salt-Mediated Asymmetric Direct Henry Reaction. J. Am. Chem. Soc. 2007, 129, 12392–12393.
- 23 He, R.; Wang, X.; Hashimoto, T.; Maruoka, K. Binaphthyl-Modified Quaternary Phosphonium Salts as Chiral Phase-Transfer Catalysts: Asymmetric Amination of β-Keto Esters. Angew. Chem. Int. Ed. 2008, 47, 9466–9468.
- 24 Wen, S.; Li, X.; Lu, Y. Enantioselective Alkylation of Glycine Imine Promoted by Amino-Acid-Derived Phosphonium Salts. Asian J. Org. Chem. 2016, 5, 1457–1460.
- 25 Zhu, C.-L.; Zhang, F.-G.; Meng, W.; Nie, J.; Cahard, D.; Ma, J.-A. Enantioselective Base-Free Electrophilic Amination of Benzofuran-2(3H)- ones: Catalysis by Binol-Derived P-Spiro Quaternary Phos-phonium Salts. Angew. Chem. Int. Ed. 2011, 50, 5869–5872.
- 26 Zhang, H.; He, J.; Chen, Y.; Zhuang, C.; Jiang, C.; Xiao, K.; Su, Z.; Ren, X.; Wang, T. Regio- and Stereoselective Cascade of β,ϒ-Unsaturated Ketones by Dipeptided Phosphonium Salt Catalysis: Stereospecific Construction of Dihydrofuro-Fused [2,3-b] Skeletons. Angew. Chem. Int. Ed. 2021, 60, 19860–19870.
- 27 Wang, X.; Zhou, L.; Zhang, H.; Ren, X.; Gao, G.; Wang, T. Enantioselective ϒ-Addition-Driven Cascade of β,ϒ-Unsaturated Ketones by Ion-Pair Catalysis: Access to Chiral 1,3-Dioxolochroman Scaffolds. Org. Lett. 2022, 24, 38–42.
- 28 Sano, D.; Nagata, K.; Itoh, T. Catalytic Asymmetric Hydroxylation of Oxindoles by Molecular Oxygen Using a Phase-Transfer Catalyst. Org. Lett. 2008, 10, 1593–1595.
- 29 Yang, Y.; Moinodeen, F.; Chin, W.; Ma, T.; Jiang, Z.; Tan, C.-H. Pentanidium-Catalyzed Enantioselective α-Hydroxylation of Oxindoles Using Molecular Oxygen. Org. Lett. 2012, 14, 4762–4765.
- 30
Derek Sim, S.-B.; Wang, M.; Zhao, Y. Phase-Transfer-Catalyzed Enantioselective α-Hydroxylation of Acyclic and Cyclic Ketones with Oxygen. ACS Catal. 2015, 5, 3609–3612.
10.1021/acscatal.5b00583 Google Scholar
- 31 Wang, Y.; Zheng, Z.; Lian, M.; Yin, H.; Zhao, J.; Meng, Q.; Gao, Z. Photo-organocatalytic enantioselective α-hydroxylation of β-keto esters and β-keto amides with oxygen under phase transfer catalysis. Green Chem. 2016, 18, 5493–5499.
- 32 Inukai, T.; Kano, T.; Maruoka, K. Asymmetric α-Hydroxylation of α-Aryl-δ-lactams with Molecular Oxygen under Phase-Transfer Conditions. Org. Lett. 2021, 23, 792–796.
- 33 Tang, X.-F.; Zhao, J.-N.; Wu. Y.-F.; Feng, S.-H.; Yang. F.; Yu, Z.-Y.; Meng, Q.-W. Visible-Light-Driven Enantioselective Aerobic Oxidation of β-Dicarbonyl Compounds Catalyzed by Cinchona-Derived Phase Transfer Catalysts in Batch and Semi-Flow. Adv. Synth. Catal. 2019, 361, 5245–5252.




