Generation and Applications of a Broad Atomic Oxygen Beam with a High Flux-Density via Collision-Induced Dissociation of O2
Comprehensive Summary
We detail the generation of a pulsed atomic oxygen (AO) broad beam with a high flux-density via collision-induced dissociation of O2 to support practical industrial exploitation of AOs, particularly for facilitating 2-dimenstional oxidation/etching at a fast rate of one-monolayer per second in an area ≥ 1000 cm2. This innovation fuses the following interdisciplinary concepts: (a) a high density of O+ can be produced in an electron-cyclotron-resonance (ECR) O2 plasma; (b) O+ can be extracted and accelerated with an aperture-electrode in the plasma; (c) O+ with adequate kinetic energy can initiate a cascade of gas-phase collisions in the presence of O2; (d) collision-induced dissociation of O2 yields AOs with adequate kinetic energy which can cause additional collision-induced dissociation of O2. Computational simulations of such collisions, with both ab initio molecular dynamics and direct simulation Monte Carlo methods, are used to guide the experimental generation of the proposed AO-beam. We experimentally demonstrate the highest known AO mean flux-density of about 1.5 × 1016 atoms·cm–2·s–1 in a broad-beam, and use it to oxidatively modify a self-assembled molecular layer of siloxane on a silicon wafer. In addition, we also demonstrate the growth of Al2O3 through an AO-assisted atomic layer deposition process at room temperature.
Background and Originality Content
Atomic oxygen (AO), generated by the dissociation of molecular oxygen, exhibits high chemical reactivity and has been extensively employed in investigating material oxidation and corrosion mechanisms, as well as in developing novel oxidation processes in the chemical industry.[1-6] Particularly, the generation of a broad beam of AOs for the controlled growth of a metal-oxide monolayer and the controlled oxidative-etch of an organic/carbonaceous device-constituent is highly desirable to fuel the emergency of 2-dimensional structures with a monolayer accuracy in advanced electronics, optoelectronics and other functional devices.[7-9] For example, atomic layer deposition (ALD) of metal-oxide has indeed become a crucial technique in many device-manufacturing processes, but its practical exploitation has been stalled by the difficulty in quickly purging the water vapor which is used to oxidize the metal-oxide precursor.[10-13] In principle, AO is more active than water vapor and AO can be purged quickly. In the engineering design of an AO-beam generator for practical manufacturing, the flux-density of AOs should be considerably higher than 1015 atoms·cm–2·s–1 in order to support the growth/etch of a monolayer-substance per second, with a broad beam covering an area of not less than 1000 cm2. An option of pulsing the AO-beam on and off every second further enables the precise control of monolayer-wised oxide-growth and etching per second. Such an AO-beam generator is ideal but has been generally conceived to be technically infeasible.
Historically, the development of an AO broad-beam was first quested for the facilitation of space-exploration. In brief, when space-crafts and artificial satellites operate within low Earth orbit (LEO), chemical reactions causing detrimental damage of such space-facilities occur because the O2 present in LEO dissociates into AO by solar ultraviolet irradiation and AO colliding with space-facilities traveling at a sonic speed is violent and reactive. More specially, the kinetic energy of such AO (about 5 eV) indeed matches the molecular chemical bond strength (3—5 eV) of typical materials and an AO flux of 1015 atoms·cm–2·s–1 is sufficient to cause collision-induced chemical-modification of one monolayer per second.[14] As such, the research in this specialized field has fostered the development of AO-beam equipment with controllable kinetic energy up to 5 eV and high throughput (1015 atoms·cm–2· s–1). This, in turn, can facilitate the investigation of novel oxidation mechanisms and applications by regulating the kinetic energy and throughput of AOs.
However, the development of high-throughput AO-beam equipment is stalled by numerous technical limitations and difficulties.[14, 15] First, although AOs can be readily produced by pyrolysis, photodissociation and plasma-reactions, the kinetic energy of AOs thus produced is uncontrollable and typically in a tame region of a few meV.[16-20] Technically, hyperthermal AOs with kinetic energy up to 10 eV can be generated by the well-established molecular-beam technology via high-pressure-jet acceleration of O2, with laser-dissociation of the accelerated O2.[20, 21] Practically, this costly method can typically give an AO-beam with a cross-sectional diameter not more than 1 mm, and is thus suitable for fundamental research but not for engineering of industrial exploitations. In this context, industrial engineering has turned to the adoption of the electron-cyclotron-resonance (ECR) plasma technology which is known to facilitate the most intensive ionization-induced dissociation of molecules in gas phase, including the dissociation of O2 into AOs and oxygen ions.[22] Oxygen ions thus generated are extracted from the plasma and then accelerated by an electric field to regulate their kinetic energy. They are finally converted to AOs with the desirable kinetic-energy by collision and charge- transfer with a metal-plate mounted near the exit of the oxygen ion beam extracted from the ECR plasma. This technology has indeed been used to produce an AO-beam of about a few eV in kinetic energy, with a cross-sectional beam diameter of about 20 cm. The AO-beam size is limited by the practical size of an ECR plasma source. This known demonstration of a practical broad AO-beam with a controllable kinetic energy has, nevertheless, the following deficiencies: (a) the AO flux density is limited by the ion flux density and even an ECR plasma can only support a maximum ion flux density of about 1 × 1015 ions·cm–2·s–1 and a beam of about 1000 cm2; (b) ion neutralization by charge-transfer with a metal-plate inevitably leads to ion-sputtering and metal-contamination of the AO-beam; (c) the AO-beam must be used close to the ion-exit from the plasma in order to prevent a severe flux-density drop caused by broadening of the ion beam and AO-beam. The placement of a workpiece-specimen close to a plasma which gives strong ultraviolet radiation and excites atoms/ molecules, however, risks detrimental damages of the specimen.
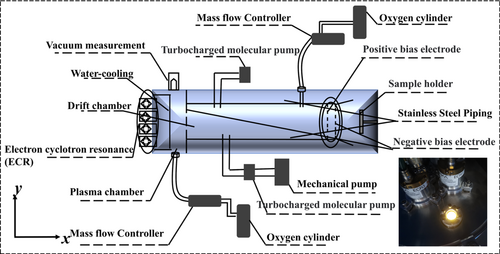
Here, an innovative and practical method is reported to overcome the deficiencies of this AO-beam technology. The present innovation articulates the science and technology of converting a broad ECR-based oxygen-ion beam with a flux of 1 × 1015 ions·cm–2· s–1 to a broad AO beam with a flux of 1.5 × 1016 atoms·cm–2·s–1 via collision-induced dissociation of O2 into AOs, as shown in Figure 1. In this AO generator, one ion injection with a kinetic energy of 300 eV initiates many collision cascades in which on the average about 130 eV of the 300 eV injection-energy is used to drive about 24 O2 dissociations each costing a dissociation energy of about 5.1 eV. Since ion extraction can be easily pulsed, the resultant AO flux can also be engineered with a pulsing frequency not less than one on/off cycle per second. The objective is the facilitation of industrial exploitation of AO surface-modification and AO-assisted metal-oxide deposition, to realize the rising demands of layer-by-layer surface-modification/deposition with a controlled operation of one atom/molecular monolayer per second, with a scalable modification/deposition area of ≥ 1000 cm2. In the following sections, the fundamental concepts of AO generation by gas-phase dissociative collisions initiated by oxygen ions extracted and accelerated from an oxygen plasma are explained by ab initio molecular dynamics (AIMD) of the kinematics in dissociative O+-O2, O-O2 and O2-O2 paired-collisions, particularly to pin-point the threshold kinetic energy required for O2 dissociation in different collision- impact trajectories. With these critical attributes, direct simulation Monte Carlo (DSMC) method of the generation of a high-flux AO-beam by extracting O+ from a plasma into a low-pressure O2 atmosphere is detailed. The simulations are then translated into the engineering designs of a practical AO-beam generator, as shown in Figure 1, with an AO flux density more than 1016 atoms· cm–2·s–1 and a beam ≥ 1000 cm2 at the proximity of the generator exit, in a pulse-operation mode with a pulsing frequency as high as one on/off cycle per second. The generator comprises an array of 2-by-2 ECR plasma sources, with a total power of 800 W. These functional features are demonstrated and validated. More specifically, the oxidative modification of a self-assembled molecular layer of alkyl silane on the surface of an oxidized silicon wafer is also explained. Further, the ALD of alumina with a fast rate of one- deposition cycle per 2 seconds is articulated. The supplementary information (SI) provides further elaboration on the details.
Simulations
AIMD simulations
Research on collision-induced O2 dissociation can be dated back to the 1960s when the dissociation of O2 was driven by argon collisions in a shock-tube apparatus and the reduction of its optical absorption intensity was detected.[23] This seminal work confirmed that under such a macroscopic collision arrangement, the condition of near-thermal-equilibrium is satisfied and the rate constant of O2 dissociation is governed by a simple Arrhenius equation such that the dissociation probability scales exponentially with –5.1 eV/kBT, where 5.1 eV is the well-known dissociation energy of O2. In shock-tube experiments, T of 5000 to 18000 K and kBT for argon rated at 0.65 to 2.35 eV are practical. As such, simulations of such collision-induced dissociation are typically performed with classical or semi-classical methods.[24] However, the collision-induced O2 dissociation in the present work is conducted with a condition far from thermal equilibrium and must be treated microscopically, with a diligent attention of each collision event in an atomic scale.
The advances in computational hardware and software now indeed support such microscopic tracks of collisions and collision- induced dissociations. In brief, ab initio calculations of the electronic structures of all collision partners in each collision event by solving the Schrödinger equation as a function of the evolution- time with a time-division as short as 0.2f s are practically conducted. The snapshots of the coordinates, rotational/vibrational states and electronic structures of the collision partners are commonly referred as ab initio molecular dynamics (AIMD). In accord to the practical experimental setup, AIMD of some 200 representative collision events are performed by adopting O+ to model the oxygen ion extracted from an ECR oxygen plasma into a cloud of O2. Although O2+ ions are also extracted, the practical operation of the experimental AO generator always injects oxygen ions at 300 eV into a cloud of O2 and a 300 eV O2+ collides with O2 which is expected to effectively convert all injected O2+ into O+ and O. Hence, AIMD with O+ as bullets is reasonable. Some exemplary collision events tracked by AIMD are depicted in Figures 2—3, with more exemplars shown in Figure S1.
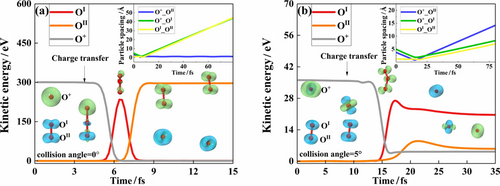
For example, the AIMD computational results for 300 eV O+ colliding O2 along the molecular axis of O2 (collision angle at 0°) are summarized in Figure 2(a). Briefly, the closest encounter between the projectile and the OI atom of O2 occurs near 6 fs, and prior to that the projectile is traveling with not any force influence until the weak van de Waals interactions prior to 3.5 fs. At 3.5 fs, the wavefunction of O+ starts interacting with that of O2 and charge exchange occurs, with an electron from the highest occupied states of O2 tunneling to the lowest unoccupied state of O+. This process leads to a transfer of potential energy between the projectile and its colliding partner via electron tunneling, and virtually induces no change of the kinematics. Then, shortly after this, the encounter of O and O2+ becomes repulsive and the wavefunction of O starts mixing with that of O2+, to form a transient molecular species which retains a configuration of O3+ in the time duration of about 5.5—7 fs. In this duration, kinetic energy and molecular potential interchange in a rather peculiar way. Apparently, at about 6.7 fs, the most stable O3+ species is formed and it flies with a kinetic energy of 300 eV. Still, this most stable O3+ is repulsive in nature and quickly dissociates into O-OI+ and OII at about 7.5 fs. Hence, although the collision is violent, the process ends with one AO and an O2+, with no generation of any additional AO.
To further illustrate the collision events in an atomic scale, the iso-surface evolutions of the spin density distributions of this system comprising three oxygen atoms are tracked and depicted in Figure 2. For example, Figure 2(a) shows, at 0 fs, two blue iso-surfaces signifying the presence of two spin-identical electrons in the px and py orbitals of the spin-polarized oxygen molecule. The spatial distributions of spin density undergo significant changes throughout the collision process. As such, these pictorial configurations also provide the most exact descriptions of all reaction intermediates in the production process of AOs.
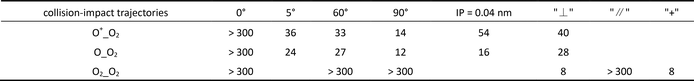
Although the violent head-on collision with a collision angle of 0° does not yield any AO generation, a slight change in collision angle causes some surprising changes. For example, when the collision angle is changed merely by 5 degrees, the shear-like adjustment of the violent collision effectively causes the dissociation of the transient repulsive molecular species of O3+ into 2AOs and an O+. Additional AIMD calculations reveal that the production of AOs in this collision configuration of “5 degrees” can proceed as long as the incoming O+ carries a kinetic energy more than 36 eV, as shown in Figure 2(b). In this case, the O+ bullet of 36 eV changes to O at about 9 fs and merges with the resultant O2+ to form a transient O3+ which survives in the duration of about 12 fs—17 fs. At 20 fs, an AO and a highly excited O2+ are formed. At 25 fs, the excited O2+ dissociates into O and O+. Clearly, the inset of Figure 2(b) illustrates that the separations among all three atomic centers are all increasing with time.
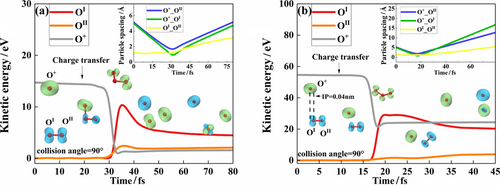
In the collision configuration when an 14 eV O+ flies head-on towards the OI atom of O2 with a velocity perpendicular to the O2 molecular axis (collision angle at 90°), a repulsive and transient intermediate, O3+, is formed and survived for the duration of about 28 fs—50 fs. But the intermediate formed by three atomic centers eventually dissociates into 2AOs and an O+ because the three atomic centers slowly move away from each other. The threshold kinetic energy of the bullet O+ for collision-induced dissociation of O2 is 14 eV for this particular collision configuration. The AIMD results are summarized in Figure 3(a). By moving the flight path of O+ merely 0.04 nm away from the OI center of O2, the slightly change in the collision impact parameter (therein referred as IP) causes a large increase from 14 to 54 eV in the dissociation threshold in reference to the kinetic energy of the O+ bullet, as shown in Figure 3(b). A comparison of the head-on case in Figure 3(a) and the IP at 0.04 nm case in Figure 3(b) validates the intuitive expectation that the kinematic change of OI which is the principal collision partner hit by the O+ bullet is less “sloppy” in the case of the head-on collision case.
|
For example, in R1, a hyperthermal O+ with 300 eV violently collides with an O2 and this leads to the dissociation of O2 into two hyperthermal AOs. With a sufficiently high kinetic energy, a resultant hyperthermal atomic oxygen may again cause the collision-induced dissociation of an O2, a reaction which yields three hyperthermal AOs. In R3, charge transfer between O+ and O2 is noted, together with kinetic energy transfer. Similarly, charge transfer between an O2+* and an O2 is noted in R4, together with kinetic energy transfer. In R5, an O2+* with a sufficiently high kinetic energy causes an extremely violent collision-induced dissociation of itself and the collided O2, with the production of four hyperthermal AOs. In comparison, R6 depicts a less violent event with only one case of dissociation. R7 and R8 are counterparts of R5 and R6, with O2* replacing O2+* as the “bullet”. The present work tracks all these reactions and statistically sizes up the essences in the AO generation by injecting 300 eV O+ into a cloud of O2 at 0.14 Pa.
DSMC method
In reference to the experimental design of an AO generator illustrated in Figure 1, the well-known direct simulation Monte Carlo (DSMC) method is adopted to track the cascades of collisions induced by the injection of 300 eV O+ into the tube chamber of the AO generator, with an O2 pressure of 0.14 Pa in the chamber.[25-30] Briefly, the conditions of rarefied gas dynamics are assumed, the Boltzmann equations describing the dynamic behaviors of the gaseous species in a single intake of O2 flowing through the tube chamber, with and without the injection of a 300 eV O+ are solved with the computational treatments extensively elucidated by Bird.[30]
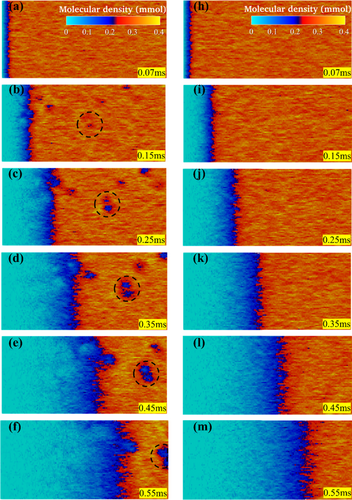
An exemplar of a collision event simulated by this DMSC approach is depicted in Figure 4, with the snapshots in the right column representing the process of a pulse of O2 flowing through the collision chamber modeled as a tube. This pulse of O2 flowing through the collision chamber is assumed to have an average molecular density of 0.4 × 10–3 mol/L. The exact molecular density distribution at each geometric location at a given time is random, and can be higher or lower than this average molecular density due to Brownian motions. The pumping operation moves this pulse of O2 at a speed of about 80 cm per 0.65 ms. In comparison, the snapshots in the left column depict the evolutions of collision cascades induced by the activation of the O+ injection electrode. More specifically, the left snapshot of Figure 4(a) shows a blue spot (a drop in molecular density) at the middle section of the tube at the snapshot time of 0.07 ms. Since in the presence of an average molecular density of 0.4 × 10–3 mol/L of O2, the average collision mean-free-path of O is about 8 cm, the location of this blue spot at the middle-section of the tube (40 cm from the left tube-opening) implies that the injected O+ has gone through about 3 collisions with large impact-parameters, i.e., 3 “missing- target” collisions with little energy-loss to its collision partners. At time near 0.07 ms, a head-on collision has occurred and a cascade of collisions has been initiated. Some O2 molecules engaging themselves in this first cascade of collisions are moving away from the central location of the cascade. This causes a drop in the molecular density and the evolution of the blue spot. In the left snapshot of Figure 4(a), additional cascades of collisions have been initiated by the first cascade of collisions at the snapshot time of 0.15 ms, particularly at the proximity of the first cascade of collisions but several cascades of collisions have also been randomly generated far away from the first cascade of collisions. The presence of such “follow-up” cascades of collisions all induce a drop in molecular density and can be located by those blue spots in the snapshot. While most “blue spots” are moving forward to the right exit of the tube, some “blue spots” are moving backward to the left entrance of the tube, as random backscattering is inevitable. The left snapshot of Figure 4(c) shows that the first aggregate of collision-cascades is exiting the tube at 0.25 ms. Since this aggregate of collision-cascades leaves the tube quickly, the “bullet” initiating the collisions must carry a relatively high kinetic energy; as such, dissociation-collisions may have occurred. If so, some AOs exit the tube at 0.25 ms. Similarly, the probability of AO production near the first cascade of collisions depicted by the blue spot in the left snapshot of Figure 4(a) is high. Hence, the left snapshot of Figure 4(f) predicts that a relatively high flux of AOs leaves the tube at the snapshot time of 0.55 ms.
In short, coupling AIMD and DSMC simulations confirms adequate AO generation by firing 300 eV O+ into a cloud of O2 at 0.14 Pa, and offers fundamental mechanistic understanding of the relevant dissociative and non-dissociative events in this innovation technology for generating a high flux of AOs.
Results and Discussion
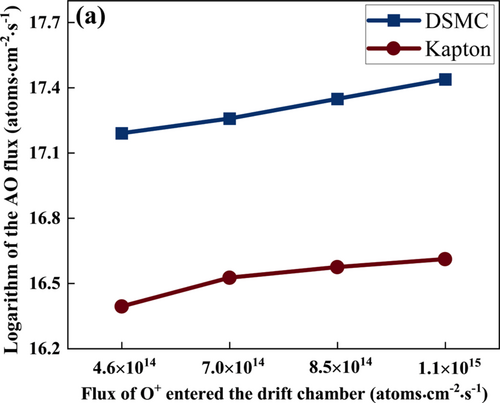
The theoretical and experimental studies provide the quantitative and qualitative results of AOs as shown in Figure 5(a). The AO flux-density increases with the increase of O+ injection flux-density. In brief, the Kapton mass loss measurements confirm that for the injection of 300 eV O+ at a flux density of 1 × 1015 ions·cm–2·s–1 into the tube chamber of the AO generator, a broad AO beam with a flux density of 1.5 × 1016 atoms·cm–2·s–1 is detected at a distance of 6 cm from the exit of the tube chamber. The Kapton mass loss measurements also confirm that the AO fluxes at the specimen-placement plane of 6 cm from the exit plane are uniform with a change of less than 10% within the central area of 30 cm in diameter. Since the tube chamber has a diameter of 30 cm, the diameter of the broad AO beam at 6 cm outside the tube exit is expected to be about 36 cm, due to random scattering of AOs. Thus, the AO flux density right at the tube exit is expected to be 2.2 × 1016 atoms·cm–2·s–1 and the AO flux is 1.53 × 1019 atoms·s–1. Since the ion flux density of 1 × 1015 ions·cm–2·s–1 is injected into the tube, with an injection diameter of 20 cm, the ion flux is 3.143 × 1017 ions·s–1. Therefore, on the average, 48.7 AOs are generated by one 300 eV O+ and roughly about 24 collision-induced dissociations of O2 are driven by each ion injection. Since the dissociation energy of O2 into 2 AOs is about 5.1 eV, the collision-induced dissociation process is effective in partitioning 300 eV of kinetic energy into a total of 122 eV to drive 24 O2 dissociations, and a total of 178 eV to raise the average kinetic energy of all collision partners. At 0.14 Pa, the mean-free-path of O2 collisions is about 8 cm; hence, the number of collisions induced by the injection of one oxygen ion at 300 eV is about 1000, with most collisions causing no O2-dissociation but merely raising the average kinetic energy of the collision partners. Therefore, the average kinetic energy of AOs at the exit of the tube chamber is roughly 178 meV. In comparison, the average kinetic energy of AOs at a temperature of 300 K is 39 meV. It is also equivalent to describe the hyperthermal AOs exiting the tube chamber have a temperature of 1369 K, or about 1100 °C.
As for the exploitation of AOs, a drop in WCA of 20° is evidence with one second of the AO beam-pulse onto the C2H5-SAM as shown in Figure S5. The surface oxidation is also revealed with the C1s XPS spectral analysis shown in Figure 6(a). In this set of data, the sample prior to any AO exposure should have a surface composition of SiO2 terminated by -C2H5 and should thus show a C 1s spectrum of those carbon atoms in -C2H5. The trace amounts of spectral signals of C—O, C=O and O—C—O chemical species are attributed to the practically inevitable adsorption of residual carbonaceous contaminants such as lubricating oils in the non-perfect ultrahigh vacuum environment during XPS analyses. A comparison of the spectral data with and without an AO dosage of 1.5 × 1016 atoms·cm–2 clearly shows a significant increase of the amounts of C—O, C=O and O—C—O chemical species on the AO-exposed sample. Thus, the carbonaceous contamination merely compromises but does not mask out the detection of AO-oxidation of the -C2H5 chemical group. Nevertheless, a quantitative analysis of the degree of oxidation of the -C2H5 chemical group is impossible due to this carbonaceous contamination. The drop in WCA of 20°, however, firms up the effectiveness of AO surface oxidation of a hydrocarbon surface/film.
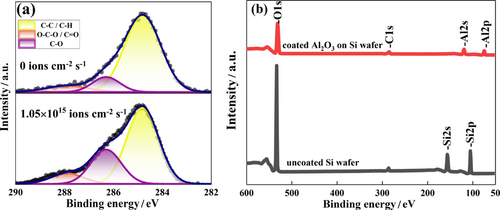
The aluminum oxide films prepared by AO-ALD exhibit a higher refractive index attributed to the absence of hydrogen atoms introduced during TMA oxidation in AO, resulting in reduced hydrogen content and consequently an elevated refractive index.[31] However, the composition of other components in AO-ALD samples generally resembles that of films prepared via thermal ALD as shown in Figure S6. Atomic layer deposition (ALD) of aluminum oxide is widely used as a protective encapsulant. Typically, one cycle of such an ALD process comprises a half cycle of TMA adsorption and a subsequent half cycle of TMA oxidation. Commonly, a flux of water molecules is used to complete the half cycle of TMA oxidation within a fraction of a second, but the complete purge of the residual water molecules is slow and typically requires a duration of 10 s or more due to the high surface sticking efficiency of water. In the present work, the flux density of 1.5 × 1016 AOs·cm–2·s–1 is expected to be sufficient for the completion of TMA oxidation in less than 1 s and the purge of AOs is virtually instantaneous. A trial run of ALD, with 1 s of TMA exposure followed by 1 s pulse of AOs, for 10 cycles indeed yields the deposition of about 7 nm of aluminum oxide on a copper disc. The XPS data summarized in Figure 6(b) confirms the fast deposition of aluminum oxide at room temperature with AO-assisted ALD. The effect on the passivation of a copper disc against thermal oxidation is demonstrated by the experimental evidence summarized in Figure S7.
Conclusions
In the present work, AIMD simulations offer clear atomistic snapshots of collision cascades induced by hiring O+ with a kinetic energy of 0—300 eV to O2. Dissociative collisions of O2 and generation of AOs are evident, and the dissociation threshold requirement of the O+ kinetic energy can be as low as 14 eV which critically depends on the exact collision configuration. Through the examination of some 200 collision configurations, a comprehensive picture of collision-induced O2 by hyperthermal O+ is obtained. The database is then used to facilitate DSMC simulations of AO production in the practical experimental design of a high- flux AO generator equipped with an ECR oxygen ion source powered to 800 W for the extraction of an ion flux density of 1 × 1015 ions·cm–2·s–1 with an extraction area of about 300 cm2, and with the ions fired into a drift tube (30 cm in diameter) containing O2 at 0.14 Pa. In essence, DSMC simulations confirm each 300 eV O+ bullet initiates cascades of collisions with some of them having collision energy higher than the dissociation thresholds estimated by AIMD simulations. As such, a microscopic mechanistic portrait of AO generation is yielded. Subsequent experimental AO flux density measurements by the well-known Kapton erosion method gives a quantitative estimate of 49 AOs generated by one 300 eV O+ injection. Since the dissociation energy of O2 is about 5.1 eV, on the average, about 122 eV of the kinetic energy carried by each O+ bullet is used to drive collision-induced dissociations of 24 O2, with the rest of input energy raising the kinetic energy and vibrational/rotational energy of each collision partners to an average of roughly 0.18 eV, which is significantly higher than their average energy in thermal equilibrium at room temperature. As such, the experimental AO broad-beam with a beam-size of 1000 cm2 and a record-high flux density of 1.5 × 1016 atoms·cm–2·s–1 bears an average energy of 0.18 eV for each AO or an AO temperature of slightly higher than 1100 °C. When a self-assembled molecular layer of ethyl trimethyl siloxane on a silicon wafer is exposed to this AO beam for 1 s, the water contact angle drops by 20° and XPS surface analysis also confirms the surface oxidation of the hydrocarbon. In addition, the same AO-beam has also been demonstrated to facilitate the atomic layer deposition of Al2O3 with a record-fast growth rate of 7 nm per 300 s. In comparison, the typical growth with water vapor to replace AO as the oxidizing agent has a typical growth rate of 7 nm per 3000—5000 s. The refractive index of the aluminum oxide film prepared using AO-assisted ALD is higher, with a film composition akin to that achieved by thermal ALD. The present work thus articulates the science and technology of practically generating a high-flux broad-beam of AOs and demonstrates some applications of such a technology innovation.
Experimental
Design and operation of a practical high-flux-AO generator
The AO generator depicted in Figure 1 comprises four compact ECR heads each with a power of 200 W, arranged in a close-packed array as an expandable plasma source. The mesh electrodes coupled to the O2 plasma source allow for the extraction of positively charged oxygen ions which are mainly O+, with a kinetic energy of 300 eV into the drift-tube with a diameter of 30 cm. For a total power of 800 W, the experimental data in the present work show that the ion flux density can reach 1 × 1015 atoms·cm–2·s–1 at the extraction aperture with a beam of 300 cm2. While the ion flux density is practically confined to this level, the diameter of the plasma source and ion source can be expanded by incorporating more compact ECR heads. Perceivably, an array of 9 ECR heads, with a total power of 1800 W, can be used to make an ion source with a beam near 1000 cm2 with an ions flux density of 1 × 1015 ions·cm–2·s–1.
Three aperture electrodes are incorporated in the drift-tube to facilitate ion extraction into the drift tube for the production of AOs in the drift-tube and to limit ions and hot-electrons leaving the exit-end of the drift-tube for the prevention of undesirable damage of the workpiece-specimen placed at the exit of the AO generator. They are also aligned such that the ultraviolet radiation from the intense plasma is blocked.
where l and λ respectively represent the length of the drift chamber and the mean free path of O2; μ is gaseous viscosity;[32] P denotes the O2 pressure in the drift-tube; vm represents the most probable velocity of O2; KB is the Boltzmann constant; T is the temperature in the drift-tube, and m is the mass of O2. The length of the drift chamber in this work is 80 cm. In a typical operation, P is 0.14 Pa, T is 300 K and λ is 8 cm; as such, the number of non-dissociative collisions can be roughly 1000. Among these collision partners, only one carries a positive charge in the completion of all these collisions; thus, charge transfer is not particularly important. The average energy of these particles is roughly about 175 meV. Hence, they are slightly more chemically active than their counterparts at 300 K. As for AO generation, merely the first 2—4 rounds of collision-cascades initiated by a 300 eV O+ are violent enough to cause O2 dissociation. The following Kapton erosion tests reveal that on the average, 24 O2 dissociations are induced by one 300 eV oxygen ion injection.
Estimations of the AO flux-density generated by the AO generator
In the present work, the well-known Kapton mass loss method for measuring AO flux densities is adopted. In addition, a self-assembled monolayer (SAM) of ethyl trimethyl siloxane (ETS) formed on an oxidized silicon wafer is exposed to the AO beam of the AO generator for testing the AO oxidative behavior. Both the changes in hydrophilicity by water contact angle measurements (WCA) and surface composition by X-ray photoelectron spectroscopy (XPS) are characterized as a function of AO exposures. The silicon wafers with coated alumina oxide were prepared by AO-assisted ALD and thermal ALD. The composition and refractive index of the films are compared and analyzed. The experimental details are included in SI.
Acknowledgement
We thank the financial support from National Natural Science Foundation of China (NSFC No. 22008007) and Scientific and Technological Innovation Foundation of Shunde Graduate School, USTB (BK21BE010) and Foshan Science and technology Innovation Project (No. 2018IT100363). The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.





