Pd-Catalyzed Dienylation of Propargylic Esters Enabling Highly Stereoselective Synthesis of Danishefsky-Type Trisubstituted Dienes
Chen Zhou
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorMengfu Dai
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorXiaoyu Yin
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorMingyue Zhang
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorWeijin Gu
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorCorresponding Author
Liang-An Chen
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]Search for more papers by this authorChen Zhou
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorMengfu Dai
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorXiaoyu Yin
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorMingyue Zhang
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorWeijin Gu
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
Search for more papers by this authorCorresponding Author
Liang-An Chen
Jiangsu Collaborative Innovation Center of Biomedical Functional Materials, Jiangsu Key Laboratory of New Power Batteries, School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, Jiangsu, 210023 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
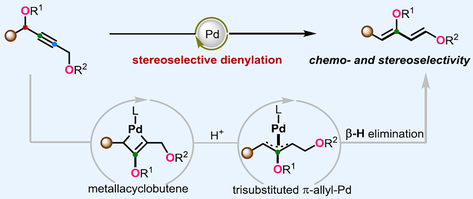
The stereochemical synthesis of highly substituted Danishefsky-type dienes remains unsolved in organic chemistry. We describe a simple and efficient approach for the stereoselective synthesis of Danishefsky-type trisubstituted dienes from readily available propargylic esters via Pd-catalyzed dienylation reaction through the key intermediate metallacyclobutene in a regio-, chemo- and stereoselective fashion. This method facilitates a broad range of challenging trisubstituted dienes with a high level of stereocontrol. The synthetic utilities of oxygenated trisubstituted dienes have been demonstrated by the downstream chemistry, which notably undergoes Diels-Alder reaction with a variety of electron-deficient dienophiles to furnish multisubstituted cyclohexenes in good yields with excellent stereoselectivity.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400195_sup_0001_supinfo.pdfPDF document, 12 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Danishefsky, S.; Kitahara, T. W. Useful Diene for the Diels-Alder Reaction. J. Am. Chem. Soc. 1974, 96, 7807–7808.
- 2
Petrzilka, M.; Grayson, J. I. Preparation and Diels-Alder Reactions of Hetero-Substituted 1,3-Dienes. Synthesis 1981, 1981, 753–786.
10.1055/s-1981-29592 Google Scholar
- 3 Fringuelli, F.; Taticchi, A. Dienes in the Diels-Alder Reaction, Wiley, New York, 1990.
- 4 Oppolzer, W. Comprehensive Organic Synthesis, Pergamon Press, New York, 1991, Vol. 5, pp. 315–400.
- 5 Ishihara, K.; Sakakura, A. Intermolecular Diels-Alder Reactions. In Comprehensive Organic Synthesis, Elsevier, New York, 2014, Vol. 5, pp. 351–408.
- 6 Zotchev, S. B. Polyene Macrolide Antibiotics and Their Applications in Human Therapy. Curr. Med. Chem. 2003, 10, 211–223.
- 7 Hubert, P.; Seibel, E.; Beemelmanns, C.; Campagne, J.-M.; de Figueiredo, R. M. Stereoselective Construction of (E,Z)-1,3-Dienes and Its Application in Natural Product Synthesis. Adv. Synth. Catal. 2020, 362, 5532–5575.
- 8 Kozmin, S. A.; Rawal, V. H. Preparation and Diels-Alder Reactivity of 1-Amino-3-Siloxy-1,3-Butadienes. J. Org. Chem. 1997, 62, 5252–5253.
- 9 Amii, H.; Kobayashi, T.; Terasawa, H.; Uneyama, K. Difluorinated Danishefsky's Diene: A Versatile C4 Building Block for the Fluorinated Six-Membered Rings. Org. Lett. 2001, 3, 3103–3105.
- 10 Yamashita, Y.; Saito, S.; Ishitani, H.; Kobayashi, S. Chiral Hetero Diels-Alder Products by Enantioselective and Diastereoselective Zirconium Catalysis. Scope, Limitation, Mechanism, and Application to the Concise Synthesis of (+)-Prelactone C and (+)-9-Deoxygoniopypyrone. J. Am. Chem. Soc. 2003, 125, 3793–3798.
- 11 Unni, A. K.; Takenaka, N.; Yamamoto, H.; Rawal, V. H. Axially Ciral Baryl diols Catalyze Highly Enantioselective Hetero-Diels-Alder Reactions through Hydrogen Bonding. J. Am. Chem. Soc. 2005, 127, 1336–1337.
- 12 Zhou, S.; Sanchez-Larios, E.; Gravel, M. Scalable Synthesis of Highly Reactive 1,3-Diamino Dienes from Vinamidinium Salts and Their Use in Diels-Alder Reactions. J. Org. Chem. 2012, 77, 3576–3582.
- 13 Xu, Z.; Zhao, J.; Zhao, D.; Yang, Z. Theoretical Investigation on Regioselectivities of Diels-Alder Reactions by Conjugated Effect. Chin. J. Chem. 2020, 38, 1696–1702.
- 14 Harada, S.; Nishida, A. Catalytic and Enantioselective Diels-Alder Reaction of Siloxydienes. Asian J. Org. Chem. 2019, 8, 732–745.
- 15 Taheri kal Koshvandi, A.; Heravi, M. M. Applications of Danishefsky's Dienes in Asymmetric Oxo-Diels-Alder Reactions. Tetrahedron: Asymmetry 2017, 28, 1506–1556.
- 16 Danishefsky, S.; Yan, C.-F.; Singh, R. K.; Gammill, R. B.; McCurry, P. M.; Fritsch, N.; Clardy, J. Derivatives of 1-Methoxy-3-Trimethylsilyloxy- 1,3-Butadiene for Diels-Alder Reactions. J. Am. Chem. Soc. 1979, 101, 7001–7008.
- 17 Danishefsky, S.; Larson, E. R.; Askin, D. Stereochemical Variations in the Cyclocondensation of Aldehydes with Siloxydienes. An Application to the Erythronolide Series. J. Am. Chem. Soc. 1982, 104, 6457–6458.
- 18 Watanabe, Y.; Shimada, N.; Anada, M.; Hashimoto, S. Enantio- and Diastereoselective Hetero-Diels-Alder Reactions between 4-Methyl- Substituted Rawal's diene and Aldehydes Catalyzed by Chiral Dirhodium(II) Carboxamidates: Catalytic Asymmetric Synthesis of(−)-cis-Aerangis Lactone. Tetrahedron: Asymmetry 2014, 25, 63–73.
- 19
Nicolaou, K. C.; Snyder, S. A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698.
10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 20 Takao, K.-I.; Munakata, R.; Tadano, K.-I. Recent Advances in Natural Product Synthesis by Using Intramolecular Diels-Alder Reactions. Chem. Rev. 2005, 105, 4779–4807.
- 21 Zhao, F.; Zhang, S.; Xi, Z. Silyl-Substituted 1,3-Butadienes for Diels–Alder Reaction, Ene Reaction and Allylation Reaction. Chem. Commun. 2011, 47, 4348–4357.
- 22 Heravi, M. M.; Vavsari, V. F. Recent Applications of Intramolecular Diels-Alder Reaction in Total Synthesis of Natural Products. RSC Adv. 2015, 5, 50890–50912.
- 23 Xue, D.-S.; Xu, M.; Zheng, C.; Yang, B.; Hou, M.; He, H.; Gao, S. Titanium-Promoted Intramolecular Photoenolization/Diels-Alder Reaction to Construct Polycyclic Terpenoids: Formal Synthesis of Mycoleptodiscin A. Chin. J. Chem. 2019, 37, 135–139.
- 24 Cai, Q. The [4+2] Cycloaddition of 2-Pyrone in Total Synthesis. Chin. J. Chem. 2019, 37, 946–976.
- 25
Dai, M.; Sun, Z.; Chen. L.-A. Palladium-Catalyzed Regiodivergent Synthesis of 1,3-Dienyl and Allyl Esters from Propargyl Esters. Angew. Chem. Int. Ed. 2022, 61, e20220383.
10.1002/anie.202203835 Google Scholar
- 26 Sun, Z.; Dai, M.; Ding, C.; Chen, S.; Chen, L.-A. Regiodivergent and Stereoselective Synthesis of Highly Substituted 1,3-Dienes via Arylative Acyloxy Migration of Propargyl Esters. J. Am. Chem. Soc. 2023, 145, 18115–18125.
- 27 Su, C.-C.; Chen, J.-T.; Lee, G.-H.; Wang, Y. Direct Approach to Palladium-mediated Cycloaddition. First Single-Crystal Structure and Convenient Synthesis of Zwitterionic η3-Trimethylenemethane Palladium from Nucleophilic Addition of Carbanions to An Allenyl Complex. J. Am. Chem. Soc. 1994, 116, 4999–5000.
- 28 Tsutsumi, K.; Ogoshi, S.; Nishiguchi, S.; Kurosawa, H. Synthesis, Structure, and Reactivity of Neutral η3-Propargyl Palladium Complexes. J. Am. Chem. Soc. 1998, 120, 1938–1939.
- 29 Chen, J.-T. Addition Reactions of Mononuclear η3-Allenyl/Propargyl Transition Metal Complexes: A New Class of Potent Organometallic Carbon Electrophiles. Coord. Chem. Rev. 1999, 190–192, 1143–1168.
- 30 Baize, M. W.; Blosser, P. W.; Plantevin, V.; Schimpff, D. G.; Gallucci, J. C.; Wojcicki, A. η3-Propargyl/Allenyl Complexes of Platinum and Palladium of the Type [(PPh3)2M(η3-CH2CCR)]+. Organometallics 1996, 15, 164–173.
- 31 Tsuji, J.; Mandai, T. Palladium-Catalyzed Reactions of Propargylic Compounds in Organic Synthesis. Angew. Chem. Int. Ed. 1996, 34, 2589–2612.
- 32 Locascio, T. M.; Tunge, J. A. Palladium-Catalyzed Regiodivergent Substitution of Propargylic Carbonates. Chem.-Eur. J. 2016, 22, 18140–18146.
- 33 O’Broin, C. Q.; Guiry, P. J. Advances in Decarboxylative Palladium-Catalyzed Reactions of Propargyl Electrophiles. J. Org. Chem. 2020, 85, 10321–10333.
- 34
Bogdos, M. K.; Stepanović, O.; Bismuto, A.; Luraschi, M. G.; Morandi, B. Mechanistically Informed Selection Rules for Competing β-Hydride and β-Heteroatom Eliminations. Nat. Synth. 2022, 1, 787–793.
10.1038/s44160-022-00145-x Google Scholar
- 35 Singh, K.; Staig, S. J.; Weaver, J. D. Weaver Facile Synthesis of Z-Alkenes via Uphill Catalysis. J. Am. Chem. Soc. 2014, 136, 5275–5278.




