Catalytic Asymmetric Synthesis of Inherently Chiral Saddle-Shaped Dibenzo[b,f][1,5]diazocines†
Jinmiao Zhou
School of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210 China
Search for more papers by this authorMengyao Tang
School of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210 China
Search for more papers by this authorCorresponding Author
Xiaoyu Yang
School of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210 China
E-mail: [email protected]Search for more papers by this authorJinmiao Zhou
School of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210 China
Search for more papers by this authorMengyao Tang
School of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210 China
Search for more papers by this authorCorresponding Author
Xiaoyu Yang
School of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
Dibenzo[b,f][1,5]diazocines are a class of eight-membered heterocycles, which exhibit unique rigid saddle-shaped structure and possess inherent chirality. In this study, we report a convenient and straightforward method for the catalytic enantioselective synthesis of these unique chiral molecules through chiral phosphoric acid-catalyzed dimerization of 2-acylbenzoisocyanates. Notably, the addition of corresponding 2-acylaniline as the co-catalyst significantly improved the efficiency of these reactions, and a simple phase separation operation resulted in products with excellent enantiopurity. Experimental studies were performed to elucidate the mechanism behind these reactions, leading to the proposal of a plausible reaction mechanism based on the study findings.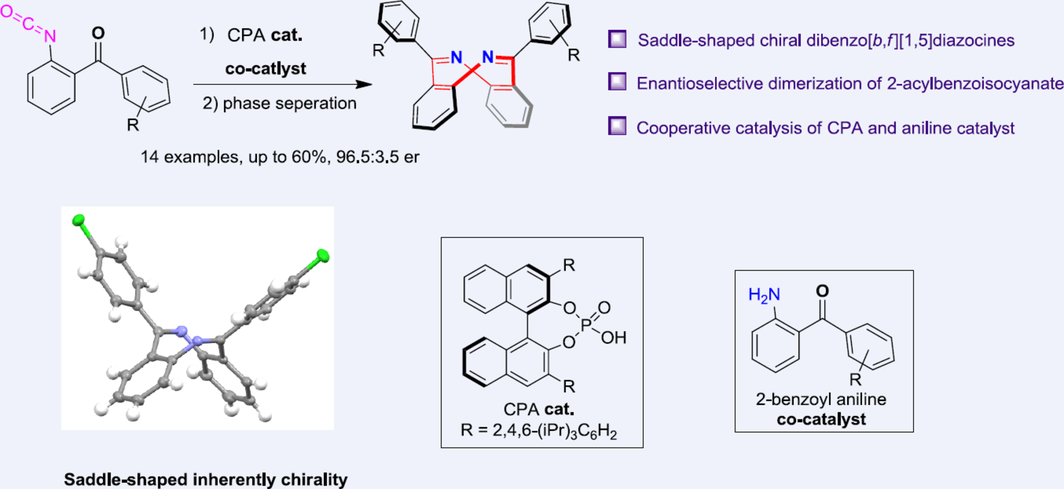
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400243-sup-0001-supinfo.pdfPDF document, 7.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Hussain, A.; Yousuf, S. K.; Mukherjee, D. Importance and synthesis of benzannulated medium-sized and macrocyclic rings (BMRs). RSC Adv. 2014, 4, 43241–43257.
- 2(a) Shiina, I. Total Synthesis of Natural 8- and 9-Membered Lactones: Recent Advancements in Medium-Sized Ring Formation. Chem. Rev. 2007, 107, 239–273;
(b) Parenty, A.; Moreau, X.; Niel, G.; Campagne, J. M. Update 1 of: Macrolactonizations in the Total Synthesis of Natural Products. Chem. Rev. 2013, 113, PR1–PR40.
10.1021/cr300129n Google Scholar
- 3(a) Allinger, N. L.; Szkrybalo, W.; DaRooge, M. A. On the Barrier to Inversion of Cyclooctatetraene. The Thermal Decomposition of Dibenzo[e,g][1,4]diazocine1. J. Org. Chem. 1963, 28, 3007–3010; (b) Lenev, D. A.; Lyssenko, K. A.; Golovanov, D. G.; Weingart, O.; Buß, V.; Kostyanovsky, R. G. Cyclic diphenic hydrazide: crystal structure, resolution, absolute configuration, and enantiomerization pathway investigation. Tetrahedron: Asymmetry 2004, 15, 537–543; (c) Olszewska, T.; Gdaniec, M.; Połoński, T. Planar Chiral Dianthranilide and Dithiodianthranilide Molecules: Optical Resolution, Chiroptical Spectra, and Molecular Self-Assembly. J. Org. Chem. 2004, 69, 1248–1255.
- 4(a) Han, J.-W.; Chen, J.-X.; Li, X.; Peng, X.-S.; Wong, H. N. C. Recent Developments and Applications of Chiral Tetraphenylenes. Synlett 2013, 24, 2188–2198;
(b) Han, J.-W.; Peng, X.-S.; Wong, H. N. C. Synthesis of tetraphenylene derivatives and their recent advances. Natl. Sci. Rev. 2017, 4, 892–916;
(c) Rapson, W. S.; Shuttleworth, R. G.; van Niekerk, J. N., 89. Benzcyclooctatetraenes. Part III. Diphenylene and tetraphenylene. J. Chem. Soc. 1943, 326–327;
10.1039/jr9430000326 Google Scholar(d) Wen, J.-F.; Hong, W.; Yuan, K.; Mak, T. C. W.; Wong, H. N. C. Synthesis, Resolution, and Applications of 1,16-Dihydroxytetraphenylene as a Novel Building Block in Molecular Recognition and Assembly. J. Org. Chem. 2003, 68, 8918–8931; (e) Peng, H.-Y.; Lam, C.-K.; Mak, T. C. W.; Cai, Z.; Ma, W.-T.; Li, Y.-X.; Wong, H. N. C. Chiral Rodlike Platinum Complexes, Double Helical Chains, and Potential Asymmetric Hydrogenation Ligand Based on “Linear” Building Blocks: 1,8,9,16-Tetrahydroxytetraphenylene and 1,8,9,16-Tetrakis(diphenylphosphino)tetraphenylene. J. Am. Chem. Soc. 2005, 127, 9603–9611; (f) Huang, H.; Stewart, T.; Gutmann, M.; Ohhara, T.; Niimura, N.; Li, Y.-X.; Wen, J.-F.; Bau, R.; Wong, H. N. C. To Flip or Not To Flip? Assessing the Inversion Barrier of the Tetraphenylene Framework with Enantiopure 2,15-Dideuteriotetraphenylene and 2,7-Dimethyltetraphenylene. J. Org. Chem. 2009, 74, 359–369; (g) Guo, J.; Ma, H.-R.; Xiong, W.-B.; Fan, L.; Zhou, Y.-Y.; Wong, H. N. C.; Cui, J.-F. Iridium-Catalyzed Enantioselective Alkynylation and Kinetic Resolution of Alkyl Allylic Alcohols. Chem. Sci. 2022, 13, 4608–4615.
- 5(a) Tang, M.; Yang, X. Catalytic Enantioselective Synthesis of Inherently Chiral Molecules: Recent Advances. Eur. J. Org. Chem. 2023, e202300738; (b) Li, J.-H.; Li, X.-K.; Feng, J.; Yao, W.; Zhang, H.; Lu, C.-J.; Liu, R.-R. Organocatalytic Enantioselective Synthesis of Seven-Membered Ring with Inherent Chirality. Angew. Chem. Int. Ed. 2024, 63, e202319289; (c) Tahara, Y.-k.; Matsubara, R.; Mitake, A.; Sato, T.; Kanyiva, K. S.; Shibata, T. Catalytic and Enantioselective Synthesis of Chiral Multisubstituted Tribenzothiepins by Intermolecular Cycloadditions. Angew. Chem. Int. Ed. 2016, 55, 4552–4556; (d) Wang, X.; Wang, C.; Luo, Y.; Li, J.; Gan, C.; Luo, S.; Zhu, Q. Enantioselective synthesis of inherently chiral 9-benzylidene-9H-tribenzo[a,c,e][7]annulene and its application as a ligand platform. Chem. Catal. 2024, 4, 100904; (e) Huang, S.; Wen, H.; Tian, Y.; Wang, P.; Qin, W.; Yan, H. Organocatalytic Enantioselective Construction of Chiral Azepine Skeleton Bearing Multiple-Stereogenic Elements. Angew. Chem. Int. Ed. 2021, 60, 21486–21493.
- 6 Shibata, T.; Chiba, T.; Hirashima, H.; Ueno, Y.; Endo, K. Catalytic Enantioselective Synthesis of Chiral Tetraphenylenes: Consecutive Inter- and Intramolecular Cycloadditions of Two Triynes. Angew. Chem. Int. Ed. 2009, 48, 8066–8069.
- 7 Luo, Y.; Cheng, S.; Peng, Y.; Wang, X.; Li, J.; Gan, C.; Luo, S.; Zhu, Q. A New Saddle-Shaped Aza Analog of Tetraphenylene: Atroposelective Synthesis and Application as a Chiral Acylating Reagent. CCS Chem. 2022, 4, 2897–2905.
- 8 Luo, Y.; Wang, X.; Hu, W.; Peng, Y.; Wang, C.; Yu, T.; Cheng, S.; Li, J.; He, Y.; Gan, C.; Luo, S.; Zhu, Q., Inherently Chiral 6,7-Diphenyldibenzo[e,g][1,4]diazocine: Enantioselective Synthesis and Application as a Ligand Platform. CCS Chem. 2023, 5, 982–993.
- 9(a) Zhang, D.; Zhou, J.; Qin, T.; Yang, X. Asymmetric synthesis of saddle-shaped eight-membered azaheterocycles via (dynamic) kinetic resolution. Chem. Catal. 2024, 4, 100827; (b) Luo, Y.; Luo, S.; Zhu, Q. Inherently chiral azaheterocycles: Asymmetric synthesis via (dynamic) kinetic resolution. Chem. Catal. 2024, 4, 100864..
- 10 Ramle, A. Q.; Tiekink, E. R. T. Synthetic strategies and diversification of dibenzo[1,5]diazocines. Org. Biomol. Chem. 2023, 21, 2870–2888.
- 11 Duncan, G. W.; Lyster, S. C.; Wright, J. B. Reproductive Mechanisms Influenced by a Diazocine. Proceedings of the Society for Experimental Biology and Medicine 1965, 120, 725–728.
- 12(a) Zhu, J.; Han, Y.; Ni, Y.; Li, G.; Wu, J. Facile Synthesis of Nitrogen-Doped [(6.)m8]nCyclacene Carbon Nanobelts by a One-Pot Self-Condensation Reaction. J. Am. Chem. Soc. 2021, 143, 2716–2721; (b) Zhu, J.; Han, Y.; Ni, Y.; Wu, S.; Zhang, Q.; Jiao, T.; Li, Z.; Wu, J. Facile Synthesis of a Fully Fused, Three-Dimensional π-Conjugated Archimedean Cage with Magnetically Shielded Cavity. J. Am. Chem. Soc. 2021, 143, 14314–14321.
- 13(a) Hwang, J.; Park, J.; Kim, Y. J.; Ha, Y. H.; Park, C. E.; Chung, D. S.; Kwon, S.-K.; Kim, Y.-H. Indolo[3,2-b]indole-Containing Donor–Acceptor Copolymers for High-Efficiency Organic Solar Cells. Chem. Mater. 2017, 29, 2135–2140; (b) Lim, J.; Kim, N. Y.; Jang, W.; An, U. S.; Kyaw, A. K. K.; Kim, Y.-H.; Wang, D. H. Selective Soxhlets extraction to enhance solubility of newly-synthesized poly(indoloindole-selenophene vinylene selenophene) donor for photovoltaic applications. Nano Converg. 2020, 7, 9.
- 14 Li, Z.-Y.; Pan, Y.; Jin, L.-L.; Yin, Y.; Yang, B.-Z.; Sun, X.-Q. Chiral exploration of 6,12-diphenyldibenzo[b,f][1,5]diazocine with stable conformation. Chirality 2017, 29, 134–139.
- 15(a) Wang, D.; Shao, Y.-B.; Chen, Y.; Xue, X.-S.; Yang, X. Enantioselective Synthesis of Planar-Chiral Macrocycles through Asymmetric Electrophilic Aromatic Amination. Angew. Chem. Int. Ed. 2022, 61, e202201064; (b) Liu, W.; Qin, T.; Xie, W.; Zhou, J.; Ye, Z.; Yang, X. Enantioselective Synthesis of Azahelicenes through Organocatalyzed Multicomponent Reactions. Angew. Chem. Int. Ed. 2023, 62, e202303430; (c) Yu, S.; Bao, H.; Zhang, D.; Yang, X. Kinetic resolution of substituted amido[2.2]paracyclophanes via asymmetric electrophilic amination. Nat. Commun. 2023, 14, 5239; (d) He, F.; Shen, G.; Yang, X. Asymmetric Aminations and Kinetic Resolution of Acyclic α-Branched Ynones. Chin. J. Chem. 2022, 40, 15–20; (e) Xie, J.; Guo, Z.; Liu, W.; Zhang, D.; He, Y.-P.; Yang, X. Kinetic Resolution of 1,2-Diamines via Organocatalyzed Asymmetric Electrophilic Aminations of Anilines. Chin. J. Chem. 2022, 40, 1674–1680.
- 16(a) Zhao, N.; Qiu, L.; Wang, X.; Li, J.; Jiang, Y.; Wan, X. Trifluoroacetic acid catalyzed dibenzodiazocine synthesis: optimization and mechanism study. Tetrahedron 2012, 68, 9665–9671; (b) Wang, X.; Li, J.; Zhao, N.; Wan, X. A Rapid and Efficient Access to Diaryldibenzo[b,f][1,5]diazocines. Org. Lett. 2011, 13, 709–711.
- 17See Supporting Information for details.
- 18 Cho, S. K.; Song, J. H.; Lee, E. J.; Lee, D.-H.; Hahn, J.-T.; Jung, D.-I. Quinolines Formation by Condensation of Heteroaromatic Ketones and 2-Aminobenzophenones under MW Irradiation. Bull. Korean Chem. Soc. 2015, 36, 2746–2749.
- 19The absolute configuration of 8a was assigned from the configuration of 2a through a proposed proximity-enabled transannular electronic delocalization cyclization mechanism: (a) Eisch, J. J.; Yu, K.; Rheingold, A. L. 6,12-Diphenyldibenzo[b,f][1,5]diazocine as an Electron-Capture Agent: Efficient Mechanistic Probe for SET Processes and Reagent for the Oxidative Dimerization of Benzylic Organometallics. Eur. J. Org. Chem. 2012, 2012, 3165–3171; (b) Eisch, J. J.; Yu, K.; Rheingold, A. L. Steric Factors in the Single-Electron Transfer Carbolithiation and Transannular Reduction of 6,12-Diphenyldibenzo[b,f] [1,5]diazocine by Organolithium Reagents. Eur. J. Org. Chem. 2014, 2014, 818–832.
- 20 Shi, J.; Zhang, L.-Z.; Pan, Y.; Feng, D.-Q.; Wu, G.-Y.; Yang, K.; Sun, X.-Q.; Li, Z.-Y. Stereoselective Mannich reaction catalyzed by tetrahydroindolo[3,2-b]indole under solvent-free conditions. Tetrahedron Lett. 2022, 109, 154128.




