Electrocatalytic Multicomponent Cascade Cross-Coupling for the Synthesis of Chalcogenosulfonates
Zhiheng Zhao
State Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou, 550025 China
Search for more papers by this authorHongyan Yan
State Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou, 550025 China
Search for more papers by this authorYaqin Zhou
State Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou, 550025 China
Search for more papers by this authorWei Xue
State Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou, 550025 China
Search for more papers by this authorCorresponding Author
Lijun Gu
State Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou, 550025 China
E-mail: [email protected]Search for more papers by this authorShengyong Zhang
School of Pharmacy, Air Force Medical University, Shaanxi, Xi’an, Shaanxi, 710032 China
Search for more papers by this authorZhiheng Zhao
State Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou, 550025 China
Search for more papers by this authorHongyan Yan
State Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou, 550025 China
Search for more papers by this authorYaqin Zhou
State Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou, 550025 China
Search for more papers by this authorWei Xue
State Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou, 550025 China
Search for more papers by this authorCorresponding Author
Lijun Gu
State Key Laboratory of Green Pesticide, Key Laboratory of Green Pesticide and Agricultural Bioengineering Ministry of Education, Center for R&D of Fine Chemicals of Guizhou University, Guiyang, Guizhou, 550025 China
E-mail: [email protected]Search for more papers by this authorShengyong Zhang
School of Pharmacy, Air Force Medical University, Shaanxi, Xi’an, Shaanxi, 710032 China
Search for more papers by this authorComprehensive Summary
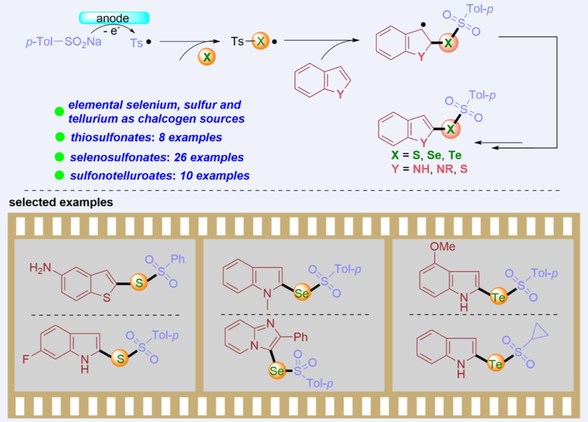
An electrocatalytic multicomponent cascade cross-coupling for the synthesis of chalcogenosulfonates has been established. This approach does not require the use of transition metals, acids, and external oxidants. The gentle conditions and tolerance to a wide variety of functional groups permit the derivatization of complex indoles.
Supporting Information
| Filename | Description |
|---|---|
| cjoc_202400158_sm_suppl.pdfPDF document, 8.4 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Mampuys, P.; McElroy, C. R.; Clark, J. H.; Orru, R. V. A.; Maes, B. U. W. Thiosulfonates as Emerging Reactants: Synthesis and Applications. Adv. Synth. Catal. 2020, 362, 3–64; (b) Wu, H. Y.; Chen, X. L.; Wu, Y. D.; Wu, A. X. Rongalite as a Versatile Reagent in Organic Synthesis. Chin. J. Chem. 2023, 41, 3388–3400; (c) Ghiazza, C.; Billard, T. Synthesis, Reactivity and Activation Modes of Fluoroalkyl Thiosulfonates and Selenosulfonates. Eur. J. Org. Chem. 2021, 2021, 5571–5584; (d) Huang, S.; Xia, Z.; Lu, K.; Lu, H.; Tung, C. H.; Xu, Z. S-Trifluoroethyl Benzenesulfonothioate: A Bench-Stable Reagent for Electrophilic Trifluoroethylthiolation. Chin. J. Chem. 2020, 38, 1625–1628; (e) Zhang, J.; Lu, Q.; Liu, C.; Lei, A. Recent Advances in Oxidative Coupling Reactions. Chin. J. Org. Chem. 2015, 35, 743–759.
- 2(a) Small, L. D.; Bailey, J. H.; Cavallito, C. J. Comparison of Some Properties of Thiolsulfonates and Thiolsulfinates. J. Am. Chem. Soc. 1949, 71, 3565–3566; (b) Zottola, M. A.; Beigel, K.; Soni, S. D.; Lawrence, R. Disulfides as Cyanide Antidotes: Evidence for a New In Vivo Oxidative Pathway for Cyanide Detoxification. Chem. Res. Toxicol. 2009, 22, 1948–1953.
- 3(a) Wang, X.; Meng, J.; Zhao, D.; Tang, S.; Sun, K. Synthesis and Applications of Thiosulfonates and Selenosulfonates as Free-adical Reagents. Chin. Chem. Lett. 2023, 34, 107736; (b) Crespi, S.; Fagnoni, M. Generation of Alkyl Radicals: From the Tyranny of Tin to the Photon Democracy. Chem. Rev. 2020, 120, 9790–9833; (c) Pannecoucke, X.; Besset, T. Use of ArSO2SRf Reagents: An Efficient Tool for the Introduction of SRf Moieties. Org. Biomol. Chem. 2019, 17, 1683–1693; (d) Nogueira, C. W.; Zeni, G.; Rocha, J. B. T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6286; (e) Ge, D.; Chen, J. W.; Xu, P.; Pan, J.; Chu, X. Q. 1,n-Thiosulfonylation Using Thiosulfonates as Dual Functional Reagents. Chin. Chem. Lett. 2022, 33, 4732–4739; (f) Abdtawfeeq, T. H.; Mahmood, E. A.; Azimi, S. B.; Kadhim, M. M.; Kareem, R. T.; Charati, F. R.; Vessally, E. Direct Selenosulfonylation of Unsaturated Compounds: A Review. RSC Adv. 2022, 12, 30564–30576; (g) Wang, H.; He, M.; Li, Y.; Zhang, H.; Yang, D.; Nagasaka, M.; Lv, Z.; Guan, Z.; Cao, Y.; Gong, F.; Zhou, Z.; Zhu, J.; Samanta, S.; Chowdhury, A. D.; Lei, A. Electrochemical Oxidation Enables Regioselective and Scalable α-C(sp3)-H Acyloxylation of Sulfides. J. Am. Chem. Soc. 2021, 143, 3628–3637; (h) Li, Y.; Wang, H.; Wang, Z.; Alhumade, H.; Huang, Z.; Lei, A. Electrochemical Radical-mediated Selective C(sp3)–S bond activation. Chem. Sci. 2023, 14, 372–378.
- 4(a) Lü, S.; Wang, Z.; Gao, X.; Chen, K.; Zhu, S. 1,2-Difunctionalization of Acetylene Enabled by Light. Angew. Chem. Int. Ed. 2023, 62, e202300268; (b) Zhu, D.; Shao, X. X.; Hong, X.; Lu, L. Shen, Q. PhSO2SCF2H: A Shelf-Stable, Easily Scalable Reagent for Radical Difluoromethylthiolation. Angew. Chem. Int. Ed. 2016, 55, 15807–15811; (c) Xiao, X.; Tian, H. Y.; Huang, Y. Q.; Lu, Y. J.; Fang, J. J.; Zhou, G. J.; Chen, F. E. Atom- and Step-Economic 1,3-Thiosulfonylation of Activated Allenes with Thiosulfonates to Access Vinyl Sulfones/Sulfides. Chem. Commun. 2022, 58, 6765–6768; (d) Wang, B.; Hu, Z.; Huang, L.; Ren. X.; Gao, Q.; Wang, X. Polysulfide Synthesis via Visible-Light-Induced Heteroarene-Migratory Dithiosulfonylation Reaction. Chem. Commun. 2023, 59, 7247–7250; (e) Li, H.; Cheng, Z.; Tung, C. H.; Xu, Z. Atom Transfer Radical Addition to Alkynes and Enynes: A Versatile Gold/Photoredox Approach to Thio-Functionalized Vinylsulfones. ACS Catal. 2018, 8, 8237–8243; (f) Liu, J.; Yao, H.; Li, X.; Wu, H.; Lin, A.; Yao, H.; Xu, J.; Xu, S. Organocatalytic 1,5-Trifluoromethylthio-sulfonylation of Vinylcyclopropane Mediated by Visible Light in the Water Phase. Org. Chem. Front. 2020, 7, 1314–1320; (g) Wu, Z.; Xu, Y.; Wu, X.; Zhu, C. Synthesis of Selenoether and Thioether Functionalized Bicyclo[1.1.1]pentanes. Tetrahedron 2020, 76, 131692.
- 5(a) Shyam, P. K.; Kim, Y. K.; Lee, C.; Jang, H. Y. Copper-Catalyzed Aerobic Formation of Unstable Sulfinyl Radicals for the Synthesis of Sulfinates and Thiosulfonates. Adv. Synth. Catal. 2016, 358, 56–61; (b) Perrone, E.; Alpegiani, M.; Bedeschi, A.; Borghi, D.; Giudici, F.; Franceschi, G. Cyclic thiosulfinates and Thiosulfonates from Oxidation of the 2-Thiacephem Ring System. Synthesis of (5R)-Penems by Stereospecific Sulfur Dioxide Extrusion. J. Org. Chem. 1986, 51, 3413–3420; (c) Luu, T. X. T.; Nguyen, T. T. T.; Le, T. N.; Spanget-Larsen, J.; Duus, F. Fast and Efficient Green Synthesis of Thiosulfonate S-Esters by Microwave-Supported Permanganate Oxidation of Symmetrical Disulfides. J. Sulfur Chem. 2015, 36, 340–350; (d) Reddy, R. J.; Waheed, M.; Kumar, J. J. A Straightforward and Convenient Synthesis of Functionalized Allyl Thiosulfonates and Allyl Disulfanes. RSC Adv. 2018, 8, 40446–40453; (e) Harpp, D. N.; Ash, D. K.; Smith, R. A. Chemistry of Sulfenic Sulfonic Thioanhydrides. Solvent-Dependent Sulfur Extrusion. J. Org. Chem. 1979, 44, 4135–4140.
- 6(a) Zhang, X.; Cui, T.; Zhang, Y.; Gu. W.; Liu, P.; Sun, P. Electrochemical Oxidative Cross-Coupling Reaction to Access Unsymmetrical Thiosulfonates and Selenosulfonates. Adv. Synth. Catal. 2019, 361, 2014–2019; (b) Peng, Z.; Zheng, X.; Zhang, Y.; An, D.; Dong, W. H2O2-Mediated Metal-Free Protocol Towards Unsymmetrical Thiosulfonates from Sulfonyl Hydrazides and Disulfides in PEG-400. Green Chem. 2018, 20, 4428–4432; (c) Zhang, G. Y.; Lv, S. S.; Shoberu, A.; Zou, J. P. J. Org. Chem. 2017, 82, 9801–9807; (d) Back, T. G.; Collins, S.; Krishna, M. V. Reactions of Sulfonhydrazideswith Benzeneseleninic Acid, Selenium Halides, and Sulfur Halides. A Convenient Preparation of Selenosulfonates and Thiosulfonates. Can. J. Chem. 1987, 65, 38–42; (e) Iwata, S.; Senoo, M.; Hata, T.; Urabe, H. Synthesis of S-Aryl Arenethiosulfonates from N,N-Di(arenesulfonyl)hydrazines: Reduction of Sulfonyl Chlorides with an Organic Reagent. Heteroatom Chem. 2013, 24, 336–344; (f) Mahieu, J. P.; Gosselet, M.; Sebille, B.; Beuzard, Y. Synthesis of New Thiosulfonates and Disulfides from Sulfonyl Chlorides and Thiols. Synth. Comm. 1986, 16, 1709–1722.
- 7(a) Zhou, X. Q.; Tang, H. T.; Cui, F. H.; Liang, Y.; Li, S. H.; Pan, Y. M. Electrocatalytic Three-Component Reactions: Synthesis of Tellurium-Containing Oxazolidinone for Anticancer Agents. Green Chem. 2023, 25, 5024–5029; (b) Chivers, T.; Laitinen, R. S. Tellurium: A Maverick Among the Chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739; (c) Chen, Y.; Deng, X.; Jing, X.; Zhou, H. Tellurium-Mediated Organic Reactions. Chin. J. Org. Chem. 2020, 40, 4147–4154.
- 8(a) Lai, X. L.; Xu, H. C. Photoelectrochemical Asymmetric Catalysis Enables Enantioselective Heteroarylcyanation of Alkenes via C–H Functionalization. J. Am. Chem. Soc. 2023, 145, 18753–18759; (b) Cheng, X.; Lei, A.; Mei, T. S.; Xu, H. C.; Xu, K.; Zeng, C. C. Recent Applications of Homogeneous Catalysis in Electrochemical Organic Synthesis. CCS Chem. 2022, 4, 1120–1152; (c) Li, Z. M.; Shuai, B.; Ma, C.; Fang, P.; Mei, T. S. Nickel-Catalyzed Electroreductive Syntheses of Triphenylenes Using ortho-Dihalobenzene-Derived Benzynes. Chin. J. Chem. 2022, 40, 2335–2344; (d) Ma, C.; Fang, P.; Liu, Z. R.; Xu, S. S.; Xu, K.; Cheng, X.; Lei, A.; Xu, H. C.; Zeng, C. C.; Mei, T. S. Recent Advances in Organic Electrosynthesis Employing Transition Metal Complexes as Electrocatalysts. Sci. Bull. 2021, 66, 2412–2429; (e) Zhou, Y.; Zhao, Z.; Zeng, L.; Li, M.; He, Y.; Gu, L. Recent Advance in Organic Electrochemical Synthesis of Nitrogenous Heterocyclic Compounds Involving Haloids as Mediators. Chin. J. Org. Chem. 2021, 41, 1072–1080.
- 9(a) Gehani, A. A. M. A. E.; Maashi, H. A.; Harnedy, J.; Morrill, L. C. Electrochemical Generation and Utilization of Alkoxy Radicals. Chem. Commun. 2023, 59, 3655–3664;
(b) Shi, S. H.; Liang, Y.; Jiao, N. Electrochemical Oxidation Induced Selective C–C Bond Cleavage. Chem. Rev. 2021, 121, 485–505;
(c) Yang, K.; Feng, T.; Qiu, Y. Organo-Mediator Enabled Electrochemical Deuteration of Styrenes. Angew. Chem. Int. Ed. 2023, 62, e202312803;
(d) Jiang, Y. Xu, K.; Zeng, C. C. Electrophotocatalytic Si–H Activation Governed by Polarity-Matching Effects. CCS Chem. 2021, 3, 1911–1920;
10.31635/ccschem.021.202101010 Google Scholar(e) Wang, P.; Tang, S.; Lei, A. Electrocatalytic Oxidant-Free Dehydrogenative C-H/S-H Cross-Coupling. Angew. Chem. Int. Ed. 2017, 56, 3009–3013; (f) Zhu, C.; Ang, N. W. J.; Meyer, T. H.; Qiu, Y.; Ackermann, L. Organic Electrochemistry: Molecular Syntheses with Potential. ACS Cent. Sci. 2021, 7, 415–431; (g) Yang, Z.; Yang, D.; Zhang, J.; Tan, C.; Li, J.; Wang, S.; Zhang, H.; Huang, Z. Lei, A. Electrophotochemical Ce-Catalyzed Ring-Opening Functionalization of Cycloalkanols under Redox-Neutral Conditions: Scope and Mechanism. J. Am. Chem. Soc. 2022, 144, 13895–13902; (h) He, Y.; Zeng, L.; Li, M.; Zhang, S.; Li, G. Electrochemical Oxidative C–C Bond Cleavage of Ketones for C–N Bond Formation: A Route to Amides. J. Org. Chem. 2022, 87, 12622–12631.
- 10(a) Zhang, X.; Cui, T.; Zhao, X.; Liu, P.; Sun, P. Electrochemical Difunctionalization of Alkenes via Four-component Reactions Cascade Mumm Rearrangement: Rapid Access to Functionalized Imides. Angew. Chem. Int. Ed. 2020, 59, 3465–3469; (b) Zeng, L.; Li, J.; Gao, J.; Huang, X.; Wang, W.; Zheng, X.; Gu, L.; Li, G.; Zhang, S.; He, Y. An electrochemical oxidative multicomponent cascade annulation of ketones and amines used to produce imidazoles. Green Chem. 2020, 22, 3416–3420; (c) Lai, X. L.; Xu, H. C. Photoelectrochemical asymmetric catalysis enables enantioselective heteroarylcyanation of alkenes via C-H functionalization. J. Am. Chem. Soc. 2023, 145, 18753–18759; (d) Zhao, Z.; He, Y.; Li, M.; Xu, J.; Li, X.; Zhang, L.; Gu, L. An Electrochemical Multicomponent [3+1+1] Annulations to Synthesize Polysubstituted 1,2,4-Triazoles. Tetrahedron 2021, 87, 132111; (e) Ren, S.; Zhou, Q.; Zhou, H. Y.; Wang, L. W.; Mulina, O. M.; Paveliev, S .A.; Tang, H. T.; Terentʼev, A. O.; Pan, Y. M. Three-Component Electrochemical Aminoselenation of 1,3-Dienes. J. Org. Chem. 2023, 88, 5760–5771; (f) Lu, Y.; Mu, S. Y.; Li, H. X.; Jiang, J.; Wu, C.; Zhou, M. H.; Ouyang, W. T.; He, W. M. EtOH-Catalyzed Electrosynthesis of Imidazolidine-Fused Sulfamidates from N-Sulfonyl Ketimines, N-Arylglycines and Formaldehyde. Green Chem. 2023, 25, 5539–5542; (g) Wan, C.; Song, R. J.; Li, J. H. Electrooxidative 1,2-Bromoesterification of Alkenes with Acids and N-Bromosuccinimide. Org. Lett. 2019, 21, 2800–2803; (h) Zhang, T. T.; Luo, M. J.; Li, Y.; Song, R. J.; Li, J. H. Electrochemical Alkoxyhalogenation of Alkenes with Organohalides as the Halide Sources via Dehalogenation. Org. Lett. 2020, 22, 7250–7254.
- 11(a) Meng, Z. Y.; Feng, C. T.; Zhang, L.; Yang, Q.; Chen, D. X.; Xu, K. Regioselective C−H Phosphorothiolation of (Hetero)arenes Enabled by the Synergy of Electrooxidation and Ultrasonic Irradiation. Org. Lett. 2021, 23, 4214–4218; (b) Yu, M.; Huang, T.; Zhang, L.; Shabbir, M.; Gao, Y.; Chen, Y. H.; Yi, H.; Lei, A. Regioselective Electrochemical Radical Cascade Cyclization of Internal Alkynes to Selenated and Trifluoromethylated Dihydropyran. Sci. China Chem. 2023, 66, 3178–3185; (c) Zhang, C.; Zhou, Y.; Zhao, Z.; Xue, W.; Gu, L. An Electrocatalytic Three-Component Reaction for the Synthesis of Phosphoroselenoates. Chem. Commun. 2022, 58, 13951–13954; (d) Xiong, T. K.; Xia, Q.; Zhou, X. Q.; Cui, F. H.; Tang, H. T.; Pan, Y. M.; Liang, Y. Electrochemically Mediated Fixation of CO2: Synthesis of Functionalized Oxazolidine-2,4-Diones by Three-Component Reactions. Adv. Synth. Catal. 2023, 365, 2183–2187; (e) Sun, L.; Wang, L.; Alhumade, H.; Yi, H.; Cai, H.; Lei, A. Electrochemical Radical Selenylation of Alkenes and Arenes via Se–Se Bond Activation. Org. Lett. 2021, 23, 7724–7729.
- 12For details, see Supporting Information (Tables S1—S4).
- 13(a) Wei, W.; Zhan, L.; Gao, L.; Huang, G.; Ma, X. Research Progress of Electrochemical Synthesis of C-Sulfonyl Compounds. Chin. J. Org. Chem. 2023, 43, 17–35; (b) Zhu, J.; Chen, Z.; He, M.; Wang, D.; Li, L.; Qi, J.; Shi, R.; Lei, A. Metal-free Electrochemical C3-Sulfonylation of Imidazo[1,2-a]pyridines. Org. Chem. Front. 2021, 8, 3815–3819.




