Catechol-Formaldehyde Resin Coated CdS Core-Shell Composite as Robust Photocatalyst for Long-Term Sustainable Artificial Photosynthesis of H2O2
Yuexin Xiang
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorZhinan Xia
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorWanchao Hu
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorCorresponding Author
Cuiyan Tong
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
E-mail: [email protected] (C. Tong), [email protected] (C. Lü).Search for more papers by this authorYang Xiao
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorCorresponding Author
Changli Lü
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
E-mail: [email protected] (C. Tong), [email protected] (C. Lü).Search for more papers by this authorYuexin Xiang
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorZhinan Xia
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorWanchao Hu
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorCorresponding Author
Cuiyan Tong
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
E-mail: [email protected] (C. Tong), [email protected] (C. Lü).Search for more papers by this authorYang Xiao
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorCorresponding Author
Changli Lü
Institute of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
E-mail: [email protected] (C. Tong), [email protected] (C. Lü).Search for more papers by this authorComprehensive Summary
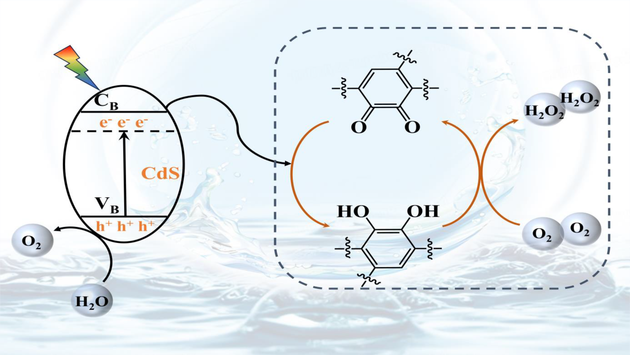
Catechol-formaldehyde resin (CFR) is very attractive for H2O2 production via the catalytic process. However, the H2O2 formation is accompanied by the oxidation of catechol groups to o-benzoquinone groups on CFR, which will cause irreversible damage to CFR and greatly limit its long-term stable catalytic activity. Herein, CdS/CFR composite photocatalyst with a core-shell structure was synthesized by hydrothermal method. The photogenerated electrons of CdS are used as a powerful driving force for the reversible redox conversion between catechol groups and o-benzoquinone groups on the CFR, which not only achieves the long-term stability of CFR-catalyzed production of H2O2, but also promotes the separation efficiency of photogenerated e– and h+ in CdS, greatly inhibiting their recombination, so as to maintain CdS stability. The H2O2 yield of CdS/CFR can accumulate to 1.65 mmol·L–1 under visible light for 6 h without sacrificial agent, which is about 3.1 and 2 times that of CdS and CFR, respectively, and CdS/CFR can persist for 10 cycles of photocatalysis (60 h). CdS/CFR also improves the yield of photocatalytic H2O2 by increasing the selectivity of H2O2 and inhibiting its decomposition. This work offers a novel tactic for expanding the application of CFR in photocatalytic generation of H2O2.
Supporting Information
| Filename | Description |
|---|---|
| cjoc_202400039_sm_suppl.pdfPDF document, 5.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Wang, L. X.; Zhang, J. J.; Zhang, Y.; Yu, H. G.; Qu, Y. H.; Yu, J. G. Inorganic metal-oxide photocatalyst for H2O2 production. Small 2022, 18, 2104561.
- 2 Hou, H. L.; Zeng, X. K.; Zhang, X. W. Production of hydrogen peroxide by photocatalytic processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376.
- 3 Meng, H.; Forooshani, P. K.; Joshi, P. U.; Osborne, J.; Mi, X.; Meingast, C.; Pinnaratip, R.; Kelley, J.; Narkar, A.; He, W. L. E.; Frost, M. C.; Heldt, C. L.; Lee, B. P. Biomimetic recyclable microgels for on-demand generation of hydrogen peroxide and antipathogenic application. Acta Biomater. 2019, 83, 109–118.
- 4 Zeng, X. K.; Liu, Y.; Hu, X. Y.; Zhang, X. W. Photoredox catalysis over semiconductors for light-driven hydrogen peroxide production. Green Chem. 2021, 23, 1466–1494.
- 5 Ahmed, M. T.; Abdullah, H.; Kuo, D. H. Photocatalytic H2O2 Generation over microsphere carbon-assisted hierarchical indium sulfide nanoflakes via a two-step one-electron pathway. ACS Appl. Mater. Interfaces 2023, 15, 29224–29235.
- 6 Bai, X. J.; Wang, X. Y.; Jia, T. Q.; Guo, L. L.; Hao, D. R.; Zhang, Z. Y.; Wu, L. Y.; Zhang, X. R.; Yang, H.; Gong, Y. W.; Li, J. Q.; Li, H. Y. Efficient degradation of PPCPs by Mo1-xS2-y with S vacancy at phase- junction: Promoted by innergenerate-H2O2. Appl. Catal. B: Environ. 2022, 310, 121302.
- 7 Li, R. Y.; Kong, W. N.; An, Z. S. Enzyme catalysis for reversible deactivation radical polymerization. Angew. Chem. Int. Ed. 2022, 61, e2022020.
- 8 Gu, M.; Lee, D.; Mun, J.; Kim, D.; Cho, H. I.; Kim, B.; Kim, W.; Lee, G.; Kim, B. S.; Kim, H. I. Solar-to-hydrogen peroxide conversion of photocatalytic carbon dots with anthraquinone: Unveiling the dual role of surface functionalities. Appl. Catal. B: Environ. 2022, 312, 121379.
- 9 Wang, Q.; Ren, L. P.; Zhang, J.; Chen, X.; Chen, C. Y.; Zhang, F.; Wang, S.; Chen, J.; Wei, J. J. Recent progress on the catalysts and device designs for (photo)electrochemical on-site H2O2 production. Adv. Energy Mater. 2023, 13, 2301543.
- 10Zhang,J. Y.; Zhang, H. C.; Cheng, M. E.; Lu, Q. Tailoring the electrochemical production of H2O2: strategies for the rational design of high-performance electrocatalysts. Small 2020, 16, 1902845.
- 11 Chen, Z.; Yao, D. C.; Chu, C. C.; Mao, S. Photocatalytic H2O2 production systems: Design strategies and environmental applications. Chem. Eng. J. 2023, 451, 138489.
- 12 Lai, C.; Xu, M. Y.; Xu, F. H.; Li, B. S.; Ma, D. S.; Li, Y. X.; Li, L.; Zhang, M. M.; Huang, D. L.; Tang, L.; Liu, S. Y.; Yan, H. C.; Zhou, X. R.; Fu, Y. K.; Yi, H. An S-scheme CdS/K2Ta2O6 heterojunction photocatalyst for production of H2O2 from water and air. Chem. Eng. J. 2023, 452, 139070.
- 13 Chen, H. B.; Xing, Y. J.; Liu, S. C.; Liang, Y. J.; Fu, J. L.; Wang, L. J.; Wang, W. Z. Mechanistic insights into efficient photocatalytic H2O2 production of 2D/2D g-C3N4/In2S3 photocatalyst by tracking charge flow direction. Chem. Eng. J. 2023, 462, 142038.
- 14 Yang, Y.; Zhu, B. C.; Wang, L. B.; Cheng, B.; Zhang, L. Y.; Yu, J. G. In-situ grown N, S co-doped graphene on TiO2 fiber for artificial photosynthesis of H2O2 and mechanism study. Appl. Catal. B: Environ. 2022, 317, 121788.
- 15 Shi, H. Y.; Li, Y.; Wang, X. F.; Yu, H. G.; Yu, J. G. Selective modification of ultra-thin g-C3N4 nanosheets on the (110) facet of Au/BiVO4 for boosting photocatalytic H2O2 production. Appl. Catal. B: Environ. 2021, 297, 120414.
- 16 Zhu, C.; Zhu, M. M.; Sun, Y.; Zhou, Y. J.; Gao, J.; Huang, H.; Liu, Y.; Kang, Z.H. Carbon-supported oxygen vacancy-rich Co3O4 for robust photocatalytic H2O2 production via coupled water oxidation and oxygen reduction reaction. ACS Appl. Energy Mater. 2019, 2, 8737–8746.
- 17 Li, Y. X.; Guo, Y.; Luan, D. Y.; Gu, X. J.; Lou, X. W. An Unlocked two-dimensional conductive Zn-MOF on polymeric carbon nitride for photocatalytic H2O2 production. Angew. Chem. Int. Ed. 2023, 62, e2023108.
- 18 Isaka, Y.; Kawase, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Two-phase system utilizing hydrophobic metal-organic frameworks (MOFs) for photocatalytic synthesis of hydrogen peroxide. Angew. Chem. Int. Ed. 2019, 58, 5402–5406.
- 19 Luo, H.; Shan, T. S.; Zhou, J. W.; Huang, L. L.; Chen, L. H.; Sa, R.; Yamauchi, Y.; You, J. M.; Asakura, Y.; Yuan, Z. H.; Xiao, H. Controlled synthesis of hollow carbon ring incorporated g-C3N4 tubes for boosting photocatalytic H2O2 production. Appl. Catal. B: Environ. 2023, 337, 122933.
- 20 Li, Y.; Zhao, Y.; Wu, J.; Han, Y. D.; Huang, H.; Liu, Y.; Kang, Z. H. Photo-charge regulation of metal-free photocatalyst by carbon dots for efficient and stable hydrogen peroxide production. J. Mater. Chem. A 2021, 9, 25453–25462.
- 21 Xiong, J.; Li, X. B.; Huang, J. T.; Gao, X. M.; Chen, Z.; Liu, J. Y.; Li, H.; Kang, B. B.; Yao, W. Q.; Zhu, Y. F. CN/rGO@BPQDs high-low junctions with stretching spatial charge separation ability for photocatalytic degradation and H2O2 production. Appl. Catal. B: Environ. 2020, 266, 118602.
- 22 Lee, D.; Park, M.; Kim, N.; Gu, M.; Kim, H. I.; Kim, B. S. Sustainable hydrogen peroxide production based on dopamine through Janus- like mechanism transition from chemical to photocatalytic reactions. J. Catal. 2022, 411, 235–244.
- 23 Zhang, X. D.; Yu, J. G.; Macyk, W.; Wageh, S.; Al-Ghamdi, A. A.; Wang, L. X. C3N4/PDA S-scheme heterojunction with enhanced photocatalytic H2O2 production performance and its mechanism. Adv. Sustain. Syst. 2023, 7, 2200113.
- 24 Shiraishi, Y.; Takii, T.; Hagi, T.; Mori, S.; Kofuji, Y.; Kitagawa, Y.; Tanaka, S.; Ichikawa, S.; Hirai, T. Resorcinol-formaldehyde resins as metal-free semiconductor photocatalysts for solar-to-hydrogen peroxide energy conversion. Nat. Mater. 2019, 18, 985–993.
- 25 Zhao, C.; Wang, X. Y.; Yin, Y. F.; Tian, W. M.; Zeng, G.; Li, H. T.; Ye, S.; Wu, L. M.; Liu, J. Molecular level modulation of anthraquinone- containing resorcinol-formaldehyde resin photocatalysts for H2O2 Production with Exceeding 1.2 % Efficiency. Angew. Chem. Int. Ed. 2023, 62, e2022183.
- 26Tan,D. M.; Zhuang, R.; Chen, R. C.; Ban, M. H.; Feng, W.; Xu, F.; Chen, X.; Wang, Q. Y. Covalent organic frameworks enable sustainable solar to hydrogen peroxide. Adv. Funct. Mater. 2024, 34, 2311655
- 27 Liao, Q. B.; Sun, Q. N.; Xu, H. C.; Wang, Y. D.; Xu, Y.; Li, Z. Y.; Hu, J. W.; Wang, D.; Li, H. J.; Xi, K. Regulating relative nitrogen locations of diazine functionalized covalent organic frameworks for overall H2O2 photosynthesis. Angew. Chem. Int. Ed. 2023, 62, e20231055.
- 28Shi,L.; Yang, L. Q.; Zhou, W.; Liu, Y. Y.; Yin, L. S.; Hai, X.; Song, H.; Ye, J. H. Photoassisted construction of holey defective g-C3N4 photocatalysts for efficient visible-light-driven H2O2 production. Small 2018, 14, 1703142.
- 29 Rui, S. Q.; Song, L. M.; Lan, J. R.; Wang, D.; Feng, S. P.; Lu, J. Y.; Wang, S. L.; Zhao, Q. F. Recent advances in carbon dots-based nanoplatforms: Physicochemical properties and biomedical applications. Chem. Eng. J. 2023, 476, 146593.
- 30 Fang, J. W.; Wang, Y.; Kurashvili, M.; Rieger, S.; Kasprzyk, W.; Wang, Q. L.; Stolarczyk, J. K.; Feldmann, J.; Debnath, T. Simultaneous hydrogen generation and exciplex stimulated emission in photobasic carbon dots. Angew. Chem. Int. Ed. 2023, 62, e2023058.
- 31Zhao,X. Y.; Li, J.; Kong, X. G.; Li, C. C.; Lin, B.; Dong, F.; Yang, G. D.; Shao, G. S.; Xue, C. Carbon dots mediated in situ confined growth of Bi clusters on g-C3N4 nanomeshes for boosting plasma-assisted photoreduction of CO2. Small 2022, 18, 2204154.
- 32 Nie, H. D.; Liu, Y.; Li, Y.; Wei, K. Q.; Wu, Z. Y.; Shi, H.; Huang, H.; Liu, Y.; Shao, M. W.; Kang, Z. H. In-situ transient photovoltage study on interface electron transfer regulation of carbon dots/NiCo2O4 photocatalyst for the enhanced overall water splitting activity. Nano Res. 2022, 15, 1786–1795.
- 33 Shiraishi, Y.; Matsumoto, M.; Ichikawa, S.; Tanaka, S.; Hirai, T. Polythiophene-doped resorcinol-formaldehyde resin photocatalysts for solar-to-hydrogen peroxide energy conversion. J. Am. Chem. Soc. 2021, 143, 12590–12599.
- 34 Shiraishi, Y.; Miura, K.; Jio, M.; Tanaka, S.; Ichikawa, S.; Hirai, T. Solar-driven generation of hydrogen peroxide on phenol-resorcinol- formaldehyde resin photocatalysts. ACS Mater. Au 2022, 2, 709–718.
- 35 Xia, C. H.; Yuan, L.; Song, H.; Zhang, C. Q.; Li, Z. M.; Zou, Y. Y.; Li, J. X.; Bao, T.; Yu, C. Z.; Liu, C. Spatial specific Janus S-scheme photocatalyst with enhanced H2O2 production performance. Small 2023, 19, 2300292.
- 36 Su, P. D.; Zhang, J. K.; Zhou, Y. H.; Wei, Z.; Zhao, S.; Yang, B.; Zhao, X.; Chen, J. Efficient photocatalytic production of hydrogen peroxide by Z-scheme resorcinol-formaldehyde resin/g-C3N4 heterostructure under visible light. Chem. Eng. J. 2023, 454, 140504.
- 37 Xiang, Y. X.; Xia, Z. N.; Hu, W. C.; Tong, C. Y.; Lü, C. L. Sustainable photocatalytic synthesis of hydrogen peroxide from catechol-formaldehyde resin microspheres modulated by nitrogen-doped carbon dots. Green Chem. 2024, 26, 1478–1487.
- 38 Wei, Z. J.; Ji, T.; Zhou, X. M.; Guo, J. W.; Yu, X.; Liu, H.; Wang, J. A. Synergistic enhancement of photocatalytic CO2 reduction by built-in electric field/piezoelectric effect and surface plasmon resonance via PVDF/CdS/Ag heterostructure. Small 2023, 19, 2304202.
- 39
Meng, Z.; Zhang, J. J.; Jiang, C. C.; Trapalis, C.; Zhang, L. Y.; Yu, J. G. Dynamics of electron transfer in CdS photocatalysts decorated with various noble metals. Small 2023, 19, 2308952.
10.1002/smll.202308952 Google Scholar
- 40 Bie, C. B.; Zhu, B. C.; Xu, F. Y.; Zhang, L. Y.; Yu, J. G. In situ grown monolayer N-doped graphene on CdS hollow spheres with seamless contact for photocatalytic CO2 reduction. Adv. Mater. 2019, 31, 1902868.
- 41 Zhang, Y.; Wu, Y. X.; Wan, L.; Ding, H. J.; Li, H. X.; Wang, X. Y.; Zhang, W. H. Hollow core-shell Co9S8@ZnIn2S4/CdS nanoreactor for efficient photothermal effect and CO2 photoreduction. Appl. Catal. B: Environ. 2022, 311, 121255.
- 42 Low, J. X.; Dai, B. Z.; Tong, T.; Jiang, C. J.; Yu, J. G. In situ irradiated X-Ray photoelectron spectroscopy investigation on a direct Z-Scheme TiO2/CdS composite film photocatalyst. Adv. Mater. 2019, 31, 1802981.
- 43 Zhao, S.; Zhao, X. Insights into the role of singlet oxygen in the photocatalytic hydrogen peroxide production over polyoxometalates- derived metal oxides incorporated into graphitic carbon nitride framework. Appl. Catal. B: Environ. 2019, 250, 408–418.
- 44 Zhang, X. M.; Liang, H. C.; Li, H. Z.; Xia, Y.; Zhu, X. H.; Peng, L.; Zhang, W.; Liu, L. L.; Zhao, T. C.; Wang, C. Y.; Zhao, Z. W.; Hung, C. T.; Zagho, M. M.; Elzatahry, A. A.; Li, W.; Zhao, D. Y. Sequential chemistry toward core-shell structured metal sulfides as stable and highly efficient visible-light photocatalysts. Angew. Chem. Int. Ed. 2020, 59, 3287–3293.
- 45 Qu, L. Y.; Liu, J. L.; Liu, Y. Y.; Zhang, G. Q.; Xu, Y. J.; Zhu, P.; Wang, Y. Z. Anchoring silver nanoparticles using catechol-derived resins: An efficient and versatile approach for producing durable antimicrobial fabrics. Prog. Org. Coat. 2023, 176, 107397.
- 46 Cao, X. J.; Guo, W. Y.; Li, A. H.; Du, J.; Du, L.; Zhang, G. J.; Liu, H. Facile synthesis of reduced graphene oxide/CdS nanowire composite aerogel with enhanced visible-light photocatalytic activity. J. Nanopart. Res. 2020, 22, 81.
- 47 Xiang, X. L.; Zhu, B. C.; Zhang, J. J.; Jiang, C. H.; Chen, T.; Yu, H. G.; Yu, J. G.; Wang, L. X. Photocatalytic H2-production and benzyl-alcohol- oxidation mechanism over CdS using Co2+ as hole cocatalyst. Appl. Catal. B: Environ. 2023, 324, 122301.
- 48 Yang, Y.; Zhu, W. J.; Shi, B. F.; Lü, C. L.; Construction of a thermo-responsive polymer brush decorated Fe3O4@catechol-formaldehyde resin core-shell nanosphere stabilized carbon dots/PdNP nanohybrid and its application as an efficient catalyst. J. Mater. Chem. A 2020, 8, 4017–4029.
- 49 Ran, J. R.; Chen, L.; Wang, D. Y.; Talebian-Kiakalaieh, A.; Jiao, Y.; Hamza, M. A.; Qu, Y.; Jing, L. Q.; Davey, K.; Qiao, S. Z. Atomic-level regulated 2D ReSe2: A universal platform boostin photocatalysis. Adv. Mater. 2023, 35, 2210164.
- 50 Li, Z. H.; Chen, T. X.; Chen, Y. F.; Chen, X. Y.; Li, L.; Kuang, S. Y.; Gao, J.; Guo, Y. X.; Lo, T. W. B.; Du, J. M. Improved H2O2 photogeneration on Rb-doped-polymeric carbon nitride via enhanced O2 adsorption. J. Mater. Chem. A 2023, 11, 5925–5936.
- 51 Ren, W. J.; Chang, Q.; Li, N.; Yang, J. L.; Hu, S. L. Carbon dots-modulated covalent triazine frameworks with exceptionally rapid hydrogen peroxide production in water. Chem. Eng. J. 2023, 451, 139035.
- 52 Cheng, J.; Wu, Y. T.; Zhang, W.; Zhang, J.; Wang, L.; Zhou, M.; Fan, F. T.; Wu, X. J.; Xu, H. X. Fully conjugated 2D sp2 carbon-linked covalent organic frameworks for photocatalytic overall water splitting. Adv. Mater. 2024, 36, 2305313.
- 53 Song, X. L.; Wei, G. F.; Sun, J.; Peng, C. D.; Yin, J. L.; Zhang, X.; Jiang, Y. L.; Fei, H. H. Overall photocatalytic water splitting by an organolead iodide crystalline material. Nat. Catal. 2020, 3, 1027–1033.
- 54 Wei, Z.; Zhao, S.; Li, W. L.; Zhao, X.; Chen, C. C.; Phillips, D. L.; Zhu, Y. F.; Choi, W. Artificial photosynthesis of H2O2 through reversible photoredox transformation between catechol and o-benzoquinone on polydopamine-coated CdS. ACS Catal. 2022, 12, 11436–11443.
- 55 Yang, C.; Wan, S. J.; Zhu, B. C.; Yu, J. G.; Cao, S. W. Calcination-regulated microstructures of donor-acceptor polymers towards enhanced and stable photocatalytic H2O2 production in pure water. Angew. Chem. Int. Ed. 2022, 61, e2022084.
- 56 Wang, K.; Li, J. P.; Liu, X. F.; Cheng, Q.; Du, Y.; Li, D.; Wang, G. H.; Liu, B. Sacrificial-agent-free artificial photosynthesis of hydrogen peroxide over step-scheme WO3/NiS hybrid nanofibers. Appl. Catal. B: Environ. 2024, 342, 123349.
- 57Zhao,Y. J. ; Liu, Y.; Wang, Z. Z.; Ma, Y. R.; Zhou, Y. J.; Shi, X. F.; Wu, Q. Y.; Wang, X.; Shao, M. W.; Huang, H.; Liu, Y.; Kang, Z. H. Carbon nitride assisted 2D conductive metal-organic frameworks composite photocatalyst for efficient visible light-driven H2O2 production. Appl. Catal. B: Environ. 2021, 289, 120035.
- 58 Liu, X.; Li, Y. D.; Lin, K .F.; Jiang, Y. Q. Construction of a novel heteropoly molybdophosphate/graphitized carbon nitride s-scheme heterostructure with enhanced photocatalytic H2O2 evolution activity. J. Colloid Interface Sci. 2024, 654, 1228–1239.
- 59 Ghoreishian, S. M.; Ranjith, K. S.; Park, B.; Hwang, S. K.; Hosseini, R.; Behjatmanesh-Ardakani, R.; Pourmortazavi, S. M.; Lee, H. U.; Son, B.; Mirsadeghi, S.; Han, Y. K.; Huh, Y. S. Full-spectrum-responsive Bi2S3@CdS S-scheme heterostructure with intimated ultrathin RGO toward photocatalytic Cr(VI) reduction and H2O2 production: Experimental and DFT studies. Chem. Eng. J 2021, 419, 129530.




