Journal list menu
Export Citations
Download PDFs
Cover Picture (Angew. Chem. Int. Ed. Engl. 7/1997)
- Page: 661
- First Published: April 18, 1997
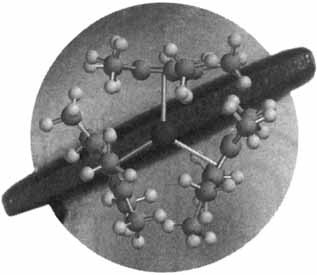
The cover picture shows the crystal structure of [U(C5Me5)3], the first 5f-element compound of this type. Previously, formation of tris(pentamethylcyclopentadienyl) complexes was limited to samarium since the only available syntheses required the special chemistry of samarium(II) precursors. Now it is possible to make these molecules from trivalent metal hydrides and tetramethylfulvalene. This new synthetic method should open up the area of [M(C5Me5)3] chemistry so that the unusual reactivity of these sterically crowded molecules can be explored. The structural diagram (generated by Michael Greci with the program Spartan 4.1.1 from Wavefunction) is superimposed on a picture of a crystal of the samarium compound [Sm(C5Me5)3], which was taken inside a specially modified Vacuum/Atmospheres glovebox by using a Panasonic videomicroscope, a McBain fiber-optic light pipe, and a Minolta Snappy video capture device. More details on the chemistry of [U(C5Me5)3] are reported by W. J. Evans et al. on pages 774 ff.
Editorial
Graphical Abstract (Angew. Chem. Int. Ed. Engl. 7/1997)
- Pages: 664-671
- First Published: April 18, 1997
Reviews
On the Trail of Xanthates: Some New Chemistry from an Old Functional Group†
- Pages: 672-685
- First Published: April 18, 1997
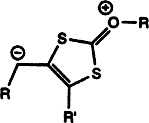
Known for nearly two centuries, yet hardly researched! The rich chemistry of xanthates leaves much to be discovered. Xanthates are cheap precursors to a great variety of radicals that can be efficiently captured in reactions free of heavy metals. S-propargyl xanthates also undergo thermal conversion into betaines of a novel type that are at the center of a host of nonradical transformations as diverse as diene formation and inversion of the configuration of secondary alcohols.
Carbenoid Complexes of Electron-Deficient Transition Metals—Syntheses of and with Short-Lived Building Blocks†
- Pages: 686-713
- First Published: April 18, 1997
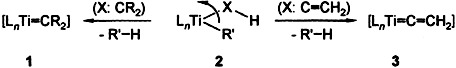
Rotatable or not rotatable? The rotation of the XH group about the MC σ-bond makes a considerable contribution to the activation energy of the conversions of 2 into carbene complexes of electron-deficient transition metals such as 1 and into the highly reactive titanaallenes 3. The multitude of reactions that 3 can undergo has increased knowledge of the chemistry of these electron-deficient transition metal complexes, which in turn has led to new discoveries about the reactivity of the alkyl and alkenyl derivatives 2 as well as of various metallacycles of titanium group metals.
Highlights
Epothilones: Promising Natural Products with Taxol-Like Activity
- Pages: 715-718
- First Published: April 18, 1997
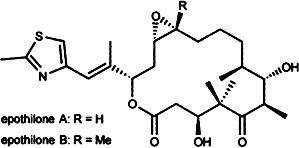
Antitumor agents possibly better than taxol might be drived from the epothilones (depicted on the right), which likewise bind to microtubules. The epothilones, which were discovered by Höfle et al. and Reichenbach et al., are easier to synthesize and more soluble in polar solvents than taxol. The results of in vivo activity studies are now eagerly awaited. The exceptional importance of these new tubulin stabilizers is reflected in the race for synthetic approaches to these compounds that has already led to the first total syntheses and several partial solutions.
Gas-Phase Complexes: Possible Prereactive Gateways for Reactions of Halogens with NH3, H2O, and H2S
- Pages: 718-721
- First Published: April 18, 1997
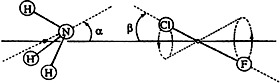
Molecular beams with the carrier gas argon contain prereactive complexes of halogens and interhalogens with NH3, H2O, and H2S, as detected by Fourier-transform microwave spectroscopy; the structures have been elucidated. The partial structures of NH3, H2O, and H2S as well as F2, ClF (cf. picture), Cl2, BrCl, and Br2 are almost the same for the complexes as for the free molecules. The weak complex bonds result from electrostatic forces.
Cross-Linked Enzyme Crystals (CLECs): Efficient and Stable Biocatalysts for Preparative Organic Chemistry†
- Pages: 722-724
- First Published: April 18, 1997
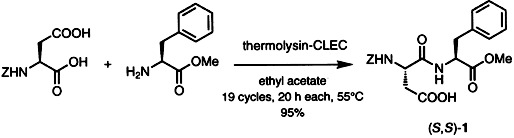
Enzyme preparations that are stable in organic solvents and in aqueous media can be obtained by covalently cross-linking enzyme crystals. The resulting exceptionally stable biocatalysts, the “cross-linked enzyme crystals” (CLECs), efficiently catalyze enzymatic peptide syntheses (see example below), esterfications, hydrolyses, CC bond-forming reactions, and reductions. Z = benzyloxycarbonyl.
Communications
A “Smart” Magnetic Resonance Imaging Agent That Reports on Specific Enzymatic Activity†
- Pages: 726-728
- First Published: April 18, 1997
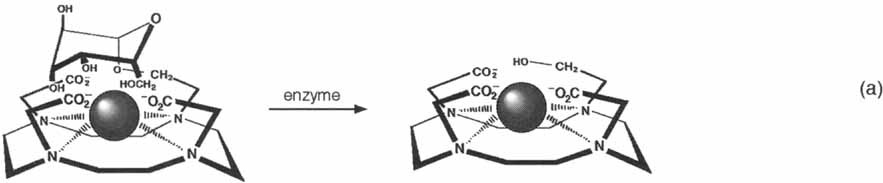
A noninvasive means to map biological structure is offered by magnetic resonance imaging (MRI). A new class of MRI contrast agents is described where the relaxivity of the complex is modified by the activity of a specific enzyme (β-galactosidase, [Eq. (a)]). This type of agent offers the promise of direct three-dimensional visualization of gene expression in the form of an acquired MR image.
Glycylglycine Rotaxanes—The Hydrogen Bond Directed Assembly of Synthetic Peptide Rotaxanes
- Pages: 728-732
- First Published: April 18, 1997
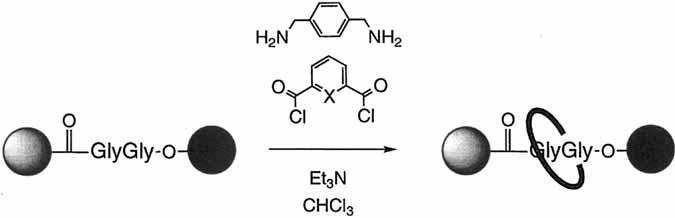
Ringing a peptide (not-so-dumb)bell: the amide groups in the glycylglycine derivative shown below provide the information required to template the formation of benzylic amide macrocycles to yield peptide [2]rotaxanes. The four key hydrogen bonds responsible for cyclization remain intact in the rotaxane in nonpolar solvents, in the solid state, and (when X = N) even in polar solvents such as [D6]DMSO and [D6]DMSO/D2O mixtures. X = CH, N; shaded sphere = Ph2CH, black sphere = Ph2CHCH2.
A Convergent Synthesis of a Carbohydrate-Containing Dendrimer†
- Pages: 732-735
- First Published: April 18, 1997
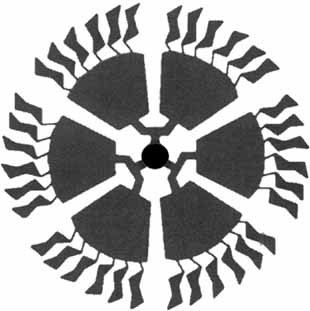
Thirty-six peripheral glucopyranosidic units are placed in the higher generation dendrimer prepared by a convergent growth strategy from dendimer wedges and an acid core (see schematic drawing on right). These compounds permit a novel entry into hitherto unknown neoglycoconjugate systems, which can be used for studying carbohydrate–protein interactions.
Supramolecular Weaving†
- Pages: 735-739
- First Published: April 18, 1997
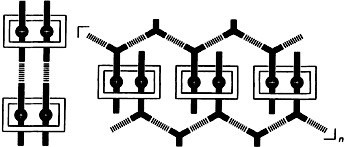
The concurrent operation of two distinct hydrogen-bonding motifs, namely the carboxyl dimer supramolecular synthon (dashed lines represent hydrogen bonds) and the threading of two secondary dialkylammonium cations (black bars) through the cavity of the ditopic macrocyclic polyether bis-p-phenylene[34]crown-10 (rectangular boxes) leads to unique superstructures. The macrocyclic polyether enforces supramolecular preorganization of the carboxyl groups, permitting formation of novel doubly encircled supermolecules and interwoven supramolecular arrays in the solid state (the diagram shows a schematic representation).
The Reactivity of the Unbridged CoSn Bond in [(η5-C5H5)(η2-C2H4)CoSn{CH[Si(CH3)3]2}2]—The First Organometallic Complexes with direct CoSnChalcogen Bonding (Chalcogen = Se, Te)†
- Pages: 739-741
- First Published: April 18, 1997
![The Reactivity of the Unbridged Co<span class='icomoon'></span>Sn Bond in [(η5-C5H5)(η2-C2H4)Co<span class='icomoon'></span>Sn{CH[Si(CH3)3]2}2]—The First Organometallic Complexes with direct Co<span class='icomoon'></span>Sn<span class='icomoon'></span>Chalcogen Bonding (Chalcogen = Se, Te)](/cms/asset/1d09ccb6-eb70-4362-8d2d-3eca80b94cf5/must001.jpg)
Butterflylike frameworks are present in the isostructural clusters 1 and 2, in which the chalcogen atom is bound as a μ3 ligand to Co and Sn. These complexes are formed by Se/Te addition to the unbridged CoSn bond of 3 at room temperature. Compounds 1 and 2 are the first defined molecular complex compounds that contain the typical semiconductor combination Sn/E (E = Se, Te) and a ferromagnetic metal, and thus they could be of interest for applications in materials science.
Asymmetric Dihydroxylation with MeO-Polyethyleneglycol-Bound Ligands†
- Pages: 741-743
- First Published: April 18, 1997
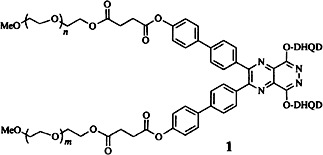
Homogenous catalysis as long as it is needed: the advantages of homogeneous and heterogeneous metal catalysis can be combined by attachment of an alkaloid ligand to polyethyleneglycol methylmonoether. Diols with up to 99% ee have been obtained in the asymmetric Sharpless dihydroxylation of olefins with the polymer modified ligand 1 (DHQD = dihydroquinidine).
A Molecular Composite Constructed in Aqueous Alkaline Solution from a Double Six-Ring Silicate and α-Cyclodextrin†
- Pages: 743-745
- First Published: April 18, 1997
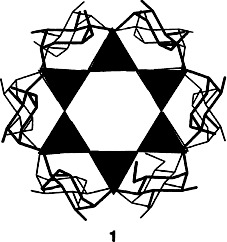
Twenty four hydrogen bonds of the type ROH…︁−OSi bind the double six-ring silicate components and the oligosaccharide α-cyclodextrin (α-CD) in K12Si12O30 · 2α-CD·36H2O (1, tetrahedral representation depicted on the right). Thus, α-cyclodextrin is is incorporated in an unusual way as a multidentate hydrogen-bond donor capable of acting as a building block of high information content in a supramolecular assembly. Compound 1 is of relevance to current discussions on biomineralization.
Ab Initio Density Funtional vs Hartree Fock Predictions for the Structure of [18]Annulene: Evidence for Bond Localization and Diminished Ring Currents in Bicycloannelated [18]Annulenes†
- Pages: 745-748
- First Published: April 18, 1997
![Ab Initio Density Funtional vs Hartree Fock Predictions for the Structure of [18]Annulene: Evidence for Bond Localization and Diminished Ring Currents in Bicycloannelated [18]Annulenes](/cms/asset/1bfc6249-df8e-4579-a53e-4aac319cc414/must001.jpg)
Threshold levels for the correct theoretical description of the delocalized [18]annulene (1) have been established with hybrid ab initio density functional computations. Analogous calculations on the bicycloannelated derivative 2 predict a clearly bond-localized structure and earmark this molecule as a new synthetic target for the study of aromaticity.
The Bond Localization Energies in the Aromatic Bismethano[14]annulenes†
- Pages: 748-750
- First Published: April 18, 1997
6-Guanidinopyranoses: Novel Carbohydrate-Based Peptidomimetics†‡
- Pages: 751-752
- First Published: April 18, 1997
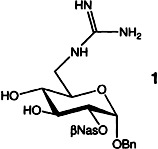
The position of the carbohydrate derivative 1 in the recognition pocket of thrombin has been determined by X-ray structure analysis of the inhibitor/thrombin complex. This confirms the structural mimicking of a peptide substrate by 1. The concept behind the synthesis of this peptidomimetic is based on attachment of the characteristic functional group of an amino acid to a carbohydrate backbone. Nas = naphthylsulfonyl.
Metal-Metal Multiple Bonds Formed Across Two Tungsten-Calix[4]arenes by a Reductive Coupling Reaction†
- Pages: 753-754
- First Published: April 18, 1997
![Metal-Metal Multiple Bonds Formed Across Two Tungsten-Calix[4]arenes by a Reductive Coupling Reaction](/cms/asset/6a76ad30-3ec2-4ef4-9d5e-905bebb84907/must001.jpg)
Tungsten–tungsten double and triple bonds are found in the dimers 1 (structure depicted on the right; calixarene substituents partly omitted) and 2, respectively, formed by the reductive coupling of the corresponding dichlorotungsten compounds. In 1 the two Na+ ions are located in the calixarene cavity; in 2 they bridge the two calixarene units. py = pyridine.
Determination of the Degree of Aggregation of Organocopper Compounds by Cryoscopy in Tetrahydrofuran†
- Pages: 755-757
- First Published: April 18, 1997
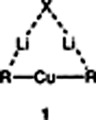
Organocuprates are mostly monomeric in solution! This is the result of the investigation of the aggregation behavior of reagents with the stoichiometry [R2Cu(X)Li2], carried out for the first time by cryoscopy in tetrahydrofuran. Thus, Gilman cuprates (X = I) and cyanocuprates (X = CN) may belong to the same structural type 1.
Total Synthesis of (–)-Epothilone B: An Extension of the Suzuki Coupling Method and Insights into Structure–Activity Relationships of the Epothilones†
- Pages: 757-759
- First Published: April 18, 1997
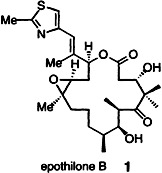
The highest biological activities displayed by epothilone-related compounds have been found for epothilone B (1) and a derivative that was prepared en route to the natural product. Key steps in the total synthesis were a Suzuki coupling providing a trisubstituted double bond in the macrocycle and its subsequent stereoselective epoxidation with dimethyldioxirane.
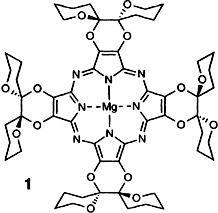
Enantiomerically Pure “Winged” Spirane Porphyrazinoctaols†‡
- Pages: 760-761
- First Published: April 18, 1997
Architectural Control in the Transition-Metal-Catalyzed Ring-Opening Polymerization of Silicon-Bridged [1]Ferrocenophanes†
- Pages: 762-764
- First Published: April 18, 1997
Long-Range Electrostatic Effects in Synthesis: Dipole-Controlled Nucleophilic Addition to a Naphthoquinone Acetal in Model Studies toward Diepoxin σ†
- Pages: 764-767
- First Published: April 18, 1997
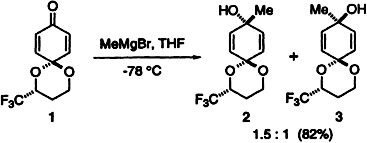
Trifluoromethylacetal groups as chiral auxiliaries enhance diastereoselectivity in nucleophilic additions to benzo- and naphthoquinone derivatives. In the methylation of 1 to give 2 and 3 facial selectivity is induced not by steric effects but by long-range electrostatic effects. This principle may be used in the design of a new class of chiral auxiliaries.
Cationic Intermediates in the Intramolecular Insertion of Alkenes into (η3-Allyl)palladium(II) Complexes†‡
- Pages: 767-769
- First Published: April 18, 1997
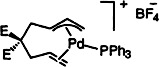
The palladium-catalyzed carbocyclization of allyl carboxylates with alkenes developed by Oppolzer proceeds smoothly via cationic complexes (depicted schematically on the right). In contrast, complexes with two donor phosphane ligands or a bidentate ligand are not productive intermediates in the cyclization process.
Structure Determination of a Complex Organic Solid from X-Ray Powder Diffraction Data by a Generalized Monte Carlo Method: The Crystal Structure of Red Fluorescein†
- Pages: 770-772
- First Published: April 18, 1997
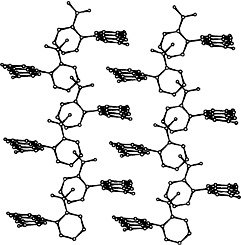
The lack of suitable crystals has meant that it has not been possible to carry out a conventional single-crystal X-ray structure analysis to determine the structure of red fluorescein. Instead, the application of a Monte Carlo method has allowed the crystal structure to be determined directly from powder X-ray diffraction data. Importantly, fluorescein was treated as a nonrigid fragment in the structure solution calculation, illustrating the generalized application of the Monte Carlo method. The molecules form layers in the crystal (with extensive CO…︁HO hydrogen bonding), and within each layer the molecules are stacked in columns (as shown on the right).
Carbohydrate-Substituted Triarylphosphanes— A New Class of Ligands for Two-Phase Catalysis†
- Pages: 772-774
- First Published: April 18, 1997
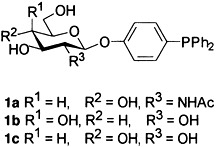
Two-phase catalysts with sugar-substituted ligands, such as glycoside–triarylphosphanes 1 a–c, reveal advantages over catalysts with the classical TPPTS ligands in Heck and Suzuki reactions. A simple and generally applicable synthesis has been developed for this new class of neutral, hydrophilic ligands. On account of thermoreversible solvation, the concentration of the two-phase catalyst in the nonpolar medium increases with increasing temperature, as shown by the Nernst distribution coefficient determined for one of the new ligands at different temperatures.
Activity of [Sm(C5Me5)3] in Ethylene Polymerization and Synthesis of [U (C5Me5)3], the First Tris(pentamethylcyclopentadienyl) 5f-Element Complex†
- Pages: 774-776
- First Published: April 18, 1997
![Activity of [Sm(C5Me5)3] in Ethylene Polymerization and Synthesis of [U (C5Me5)3], the First Tris(pentamethylcyclopentadienyl) 5f-Element Complex](/cms/asset/d1b46544-e02f-4de3-b140-1564e31b29ab/must001.jpg)
Polymerization of ethylene is initiated by [Sm(C5Me5)3], an observation that led to a new synthetic route to this SmIII compound. Tetramethylfulvalene proved to be the crucial reagent and was also used in the synthesis of [U(C5Me5)3], the first tris(pentamethylcyclopentadienyl) complex of an actinide metal (see structure on right).
Stabilization of Iron Clusters by Polyolato Ligands and Calcium Ions: An Fe14 Oxocluster from Aqueous Alkaline Solution†
- Pages: 776-779
- First Published: April 18, 1997
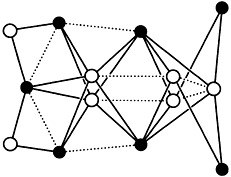
Calcium ions and organic ligands—in this case deprotonated erythritol—together are capable of reducing aggregation of oxoiron(III) species to such an extent that well-defined oxoclusters can be isolated. In the title cluster, an Fe8O32 rutile core is extended by further iron centers to yield a hematite-type microtwin (Fe14O48; arrangement of iron centers depicted on the right), which may be related to metastable oxoiron(III) phases in terms of structure and magnetism.
Integrated Chemical Process: An Extremely Concise Synthesis of Vitamin A
- Pages: 779-780
- First Published: April 18, 1997

Optimizing reactions for one set of conditions, although they normally proceed under different conditions, is a promising method for the directed development of one-pot reaction sequences. The validity of such “integrated” chemical processes has been shown by an extremely concise synthesis of vitamin A, which involves coupling of two C10 building blocks [Eq. (a)].
Combinatorial Chemistry with Laser Optical Encoding†
- Pages: 780-782
- First Published: April 18, 1997
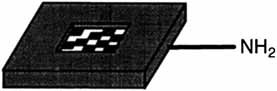
A new strategy for solid-phase combinatorial synthesis: Application of laser optical synthesis chips (shown schematically on the right; other end groups include OH, COOH, and Cl) and directed sorting allows formation and reliable characterization of libraries of small organic molecules, peptides, and oligonucleotides.
Corrigenda
Book Reviews
Book Review: Chemistry a Threat?: Green Chemistry. Designing Chemistry for the Environment. Edited by P. T. Anastas and T. C. Williamson
- Pages: 783-784
- First Published: April 18, 1997
Book Review: Multiply Bonded Main Group Metals and Metalloids. Edited by R. West and F. G. A. Stone
- Page: 784
- First Published: April 18, 1997
Book Review: The Chemistry of Heterocycles. By T. Eicher and S. Hauptmann
- Page: 785
- First Published: April 18, 1997





![The Bond Localization Energies in the Aromatic Bismethano[14]annulenes](/cms/asset/a6897471-bd38-454d-8cbd-5ce79977a0ee/must001.jpg)
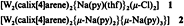
![Architectural Control in the Transition-Metal-Catalyzed Ring-Opening Polymerization of Silicon-Bridged [1]Ferrocenophanes](/cms/asset/7aa5f168-8c3d-490d-b316-ecdec4b3b4a4/must001.jpg)


































