Glycylglycine Rotaxanes—The Hydrogen Bond Directed Assembly of Synthetic Peptide Rotaxanes
Corresponding Author
Dr. David A. Leigh
Department of Chemistry, University of Manchester Institute of Science and Technology, Sackville Street, Manchester M601QD (UK), Fax: Int. code +(161) 200-4539, e-mail: [email protected]
Department of Chemistry, University of Manchester Institute of Science and Technology, Sackville Street, Manchester M601QD (UK), Fax: Int. code +(161) 200-4539, e-mail: [email protected]Search for more papers by this authorAden Murphy
Department of Chemistry, University of Manchester Institute of Science and Technology, Sackville Street, Manchester M601QD (UK), Fax: Int. code +(161) 200-4539, e-mail: [email protected]
Search for more papers by this authorDr. John P. Smart
Department of Chemistry, University of Manchester Institute of Science and Technology, Sackville Street, Manchester M601QD (UK), Fax: Int. code +(161) 200-4539, e-mail: [email protected]
Search for more papers by this authorAlexandra M. Z. Slawin
Molecular Structure Laboratory, Department of Chemistry, University of Loughborough (UK)
Search for more papers by this authorCorresponding Author
Dr. David A. Leigh
Department of Chemistry, University of Manchester Institute of Science and Technology, Sackville Street, Manchester M601QD (UK), Fax: Int. code +(161) 200-4539, e-mail: [email protected]
Department of Chemistry, University of Manchester Institute of Science and Technology, Sackville Street, Manchester M601QD (UK), Fax: Int. code +(161) 200-4539, e-mail: [email protected]Search for more papers by this authorAden Murphy
Department of Chemistry, University of Manchester Institute of Science and Technology, Sackville Street, Manchester M601QD (UK), Fax: Int. code +(161) 200-4539, e-mail: [email protected]
Search for more papers by this authorDr. John P. Smart
Department of Chemistry, University of Manchester Institute of Science and Technology, Sackville Street, Manchester M601QD (UK), Fax: Int. code +(161) 200-4539, e-mail: [email protected]
Search for more papers by this authorAlexandra M. Z. Slawin
Molecular Structure Laboratory, Department of Chemistry, University of Loughborough (UK)
Search for more papers by this authorGraphical Abstract
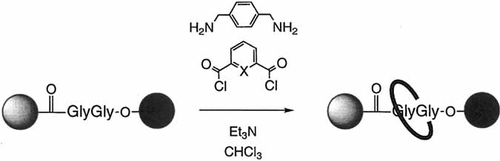
Ringing a peptide (not-so-dumb)bell: the amide groups in the glycylglycine derivative shown below provide the information required to template the formation of benzylic amide macrocycles to yield peptide [2]rotaxanes. The four key hydrogen bonds responsible for cyclization remain intact in the rotaxane in nonpolar solvents, in the solid state, and (when X = N) even in polar solvents such as [D6]DMSO and [D6]DMSO/D2O mixtures. X = CH, N; shaded sphere = Ph2CH, black sphere = Ph2CHCH2.
References
- 1 F. B. Dean, A. Stasiak, T. Koller, N. R. Cozzarelli, J. Biol. Chem. 1985, 260, 4975–4983.
- 2 D. W. Sumners, The Role of Knot Theory in DNA Research in Geometry and Topology (Eds.: C. McCrory, T. Schifrin), Marcel Dekker, New York, 1987, pp. 297–318.
- 3
C. O. Dietrich-Buchecker,
J.-P. Sauvage in
Bioorganic Chemistry Frontiers
(Ed.: H. Dugas),
Springer, Berlin,
1991, Vol.
2, pp. 195–248.
10.1007/978-3-642-76241-3_6 Google Scholar
- 4 A. D. Bates, A. Maxwell, DNA Topology, Oxford University Press, New York, 1993.
- 5 C. Liang, K. Mislow, J. Am. Chem. Soc. 1994, 116, 11189–11190.
- 6 C. Liang, K. Mislow, J. Am. Chem. Soc. 1995, 117, 4201–4213.
- 7 F. Takusagawa, S. Kamitori, J. Am. Chem. Soc. 1996, 118, 8945–8946.
- 8 C. Liang, K. Mislow, J. Am. Chem. Soc. 1994, 116, 3588–3589.
- 9 A. J. Lapthorn, D. C. Harris, A. Littlejohn, J. W. Lustbader, R. E. Canfield, K. J. Machin, F. J. Morgan, N. W. Isaacs, Nature 1994, 369, 455–461.
- 10 N. Q. McDonald, W. A. Hendrickson, Cell 1993, 73, 421–424.
- 11 M. P. Schlunegger, M. G. Grütter, J. Mol. Biol. 1993, 231, 445–458.
- 12 J. Murray-Rust, N. Q. McDonald, T. L. Blundell, M. Hosang, C. Oefner, F. Winkler, R. A. Bradshaw, Structure 1993, 1, 153–159.
- 13 N. C. Seeman, DNA Cell Biol. 1991, 10, 475–486.
- 14 J. Chen, N. C. Seeman, Nature 1991, 350, 631–633.
- 15 Y. Zhang, N. C. Seeman, J. Am. Chem. Soc. 1994, 116, 1661–1669.
- 16 B. H. Robinson, N. C. Seeman, Protein Eng. 1987, 1, 295–300.
- 17 D. B. Amabilino, J. F. Stoddart, Chem. Rev. 1995, 95, 2725–2828.
- 18
A. G. Johnston,
D. A. Leigh,
R. J. Prichardt,
M. D. Deegan,
Angew. Chem.
1995,
107, 1327–1327
10.1002/ange.19951071115 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1209–1212.
- 19
A. G. Johnston,
D. A. Leigh,
L. Nezhat,
J. P. Smart,
M. D. Deegan,
Angew. Chem.
1995,
107, 1327–1331;
10.1002/ange.19951071115 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1212–1216.
- 20 A. G. Johnston, D. A. Leigh, A. Murphy, J. P. Smart, M. D. Deegan, J. Am. Chem. Soc. 1996, 118, 10662–10663.
- 21 The principle mechanism for catenane formation proposed by Vögtle et al. [36] for the Hunter-Vögtle catenanes (binding of an acid chloride within the cavity of an amide macrocycle) does not account for the formation of benzylic amide catenanes. The binding constants of benzylic amide macrocycles with acid chlorides (< 10 M−1) are at least two orders of magnitude too small to account for catenane formation.
- 22 J. Otera, N. Dan-oh, H. Nozaki, J. Org. Chem. 1991, 56, 5307–5311.
- 23
D. A. Leigh,
K. Moody,
J. P. Smart,
K. J. Watson,
A. M. Z. Slawin,
Angew. Chem.
1996,
108, 326–331;
10.1002/ange.19961080311 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 306–310.
- 24 E. M. Arnett, E. J. Mitchell, T. S. S. R. Murty, J. Am. Chem. Soc. 1974, 96, 3875–3891.
- 25 C. A. Hunter, D. H. Purvis, Angew. Chem. 1992, 104, 779–782; Angew. Chem. Int. Ed. Engl. 1992, 31, 792–795.
- 26 The markedly different behaviour of the isophthaloyl and 2,6-pyridal rotaxanes in polar solvents is not a consequence of the stronger hydrogen bonding interactions in the pyridyl rotaxane, but rather a result of the different hydrogen bonding geometries possible with the isophthaloyl unit that are not accessible with the pyridyl system [19,25].
- 27 S. Ottens-Hildebrandt, M. Nieger, K. Rissanen, J. Rouvinen, S. Meier, G. Harder, F. Vögtle, J. Chem. Soc. Chem. Commun. 1995, 777–778.
- 28 H. Adams, F. J. Carver, C. A. Hunter, J. Chem. Soc. Chem. Commun. 1995, 809–810.
- 29 R. Taylor, O. Kennard, W. Versichel, J. Am. Chem. Soc. 1983, 105, 5761–5766.
- 30 P. Murray-Ruat, J. P. Glusker, J. Am. Chem. Soc. 1984, 106, 1018–1025.
- 31
G. A. Jeffrey,
W. Saenger,
Hydrogen Bonding in Biological Structures,
Springer, Berlin,
1991.
10.1007/978-3-642-85135-3 Google Scholar
- 32 D. A. Tomalia, H. D. Durst, Top. Curr. Chem. 1993, 165, 193–313.
- 33 A. Altomare, M. C. Burla, M. Camalli, M. Cascarano, C. Giacovazzo, A. Guagliardi, G. Polidori, J. Appl. Crystallogr., unpublished results.
- 34 Molecular Structure Corporation, TEXSAN, Texray Structure Analysis Package, 1985 and 1992, MSC 3200A Research Forest Drive, The Woodlands, TX 77381, USA.
- 35(a) F. Vögtle, M. Händel, S. Meier, S. Ottens-Hildebrandt, F. Ott, T. Schmidt, Liebigs Ann. 1995, 739–743; (b) F. Vögtle, R. Jäger, M. Händel, S. Ottens-Hildebrandt, W. Schmidt, Synthesis 1996, 353–356; (c) F. Vögtle, M. Händel, S. Ottens-Hildebrandt, Pure Appl. Chem. 1996, 68, 225–232; (d) F. Vögtle, M. Händel, R. Jäger, S. Meier, G. Harder, Chem. Eur. J. 1996, 2, 640–643; (e) R. Jäger, M. Händel, J. Harren, K. Rissanen, F. Vögtle, Liebigs Ann. 1996, 1201–1207.
- 36(a) S. Ottens-Hildebrandt, S. Meier, W. Schmidt, F. Vögtle, Angew. Chem. 1994, 106, 1818–1821; Angew. Chem. Int. Ed. Engl. 1994, 33, 1767–1770; (b) F. Vögtle, T. Dünnwald, T. Schmidt, Acc. Chem. Res. 1996, 29, 451–460.
- 37 Crystal data for 9: C62H57O8.50N8, Mr = 1050.18, clear cyrstal of dimensions 0.15 × 0.2 × 0.66 mm, monoclinic, space group P21/n (no. 14); a = 18.172(2), b = 16.874(4), c = 18.259(38) Å, β = 92.34(1)°, V = 5594(1) Å3, ρcalcd = 1.247 gcm−3, Z = 4; 8950 reflections measured, 8645 unique. Diffractometer Rigaku AFC7S, 2θmax = 120.1°, CuKα radiation, λ = 1.54178 Å, T = 296 K. The structure was solved by direct methods (SIR92) [33] and subjected to least-squares refinement (TEXSAN [34]) to yield final residuals of R = 0.061 and Rw = 0.045 for 713 parameters. All hydrogen atoms were placed in chemically reasonable positions. Crystallograhic data (excluding structure factors) for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as Supplementary publication no. CCDC-179-100060. Copies of the data can be obtained free of charge on application to The Director, CCDC, 12 Union Road, Cambridge CB21EZ, UK (fax: Int. code +(1223) 336-033; e-mail: [email protected])