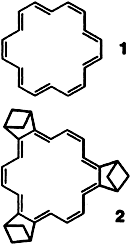
Ab Initio Density Funtional vs Hartree Fock Predictions for the Structure of [18]Annulene: Evidence for Bond Localization and Diminished Ring Currents in Bicycloannelated [18]Annulenes†
Dr. Kim K. Baldridge
San Diego Supercomputer Center, P.O. Box 85608, San Diego, CA 92186-9784 (USA)
Search for more papers by this authorCorresponding Author
Prof. Jay S. Siegel
Department of Chemistry, University of California-San Diego, La Jolla, CA 92093-0358(USA), Fax: Int. code +(619) 534-5383, e-mail: [email protected]
Department of Chemistry, University of California-San Diego, La Jolla, CA 92093-0358(USA), Fax: Int. code +(619) 534-5383, e-mail: [email protected]Search for more papers by this authorDr. Kim K. Baldridge
San Diego Supercomputer Center, P.O. Box 85608, San Diego, CA 92186-9784 (USA)
Search for more papers by this authorCorresponding Author
Prof. Jay S. Siegel
Department of Chemistry, University of California-San Diego, La Jolla, CA 92093-0358(USA), Fax: Int. code +(619) 534-5383, e-mail: [email protected]
Department of Chemistry, University of California-San Diego, La Jolla, CA 92093-0358(USA), Fax: Int. code +(619) 534-5383, e-mail: [email protected]Search for more papers by this authorThis work was supported by the U.S. National Science Foundation and the San Diego Supercomputer Center.
Graphical Abstract
Threshold levels for the correct theoretical description of the delocalized [18]annulene (1) have been established with hybrid ab initio density functional computations. Analogous calculations on the bicycloannelated derivative 2 predict a clearly bond-localized structure and earmark this molecule as a new synthetic target for the study of aromaticity.
References
- 1 K. Mislow, J. Chem. Phys., 1952, 20, 1489.
- 2 E. Vogel, H. D. Roth, Angew. Chem. 1964, 76, 145; Angew. Chem. Int. Ed. Engl. 1964, 3, 228.
- 3 R. Mitchell, Adv. Theor. Interesting Mol. 1983, 1, 135.
- 4 S. Masemune, R. T. Seidner, Chem. Commun. 1969, 542.
- 5 E. E. van Tamelen, T. L. Burkoth, J. Am. Chem. Soc. 1967, 89, 151.
- 6 E. E. van Tamelen, T. L. Burkoth, R. H. Greeley, J. Am. Chem. Soc. 1971, 93, 6120.
- 7 F. Sondheimer, Acc. Chem. Res. 1972, 5, 81.
- 8 H. Baumann, J. Am. Chem. Soc. 1978, 100, 7196.
- 9 R. C. Haddon, K. Raghavachari, J. Am. Chem. Soc. 1985, 107, 189.
- 10 E. R. Davidson, W. T. Borden, Acc. Chem. Res. 1996, 29, 67.
- 11 Y. Xie, H. F. Schaefer III, G. Liang, J. P. Bowen, J. Am. Chem. Soc. 1994, 116, 1442.
- 12 H. M. Sulzbach, P. von R. Schleyer, H. Jiao, Y. Xie, H. F. Schaefer, J. Am. Chem. Soc. 1995, 117, 1369.
- 13 P. von R. Schleyer, H. Jiao, H. M. Sulzbach, H. F. Schaefer III, J. Am. Chem. Soc. 1996, 118, 2093.
- 14 R. H. Mitchell, Y. Chen, V. S. Iver, D. Y. K. Lau, K. K. Baldridge, J. S. Siegel, J. Am. Chem. Soc. 1996, 118, 2907.
- 15 H. Jiao, P. von R. Schleyer, Angew. chem. 1996, 108, 2548; Angew. Chem. Int. Ed. Engl. 1996, 35, 2383.
- 16
M. Nendel,
K. N. Houk,
L. M. Tolbert,
E. Vogel,
H. Jiao,
P. von R. Schleyer,
Angew. Chem.
1997,
109, 760;
10.1002/ange.19971090716 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 748.10.1002/anie.199707481 Google Scholar
- 17
J. M. Schulman,
R. L. Disch,
J. Mol. Struct. (Therochem)
1991,
234, 213.
10.1016/0166-1280(91)89014-R Google Scholar
- 18 RHF calculations were performed with the aid of analytically determined gradients and the search algorithms contained within GAMESS [19], by using the 6-31G(2d, p) [20,21] and dzv(2d, p) basis sets[22]. These basis sets include two sets of six d polarization functions on all heavy atoms, and one set of p polarization functions on hydrogen atoms. DF calculations using sufficient nonlocal potentials were performed by using the DF formalism within CADPAC [23] and G94 [24], and by using the dz(ndmf, qp) basis sets [25] where n = 1, 2, m = 0, 1, and q = 0–2. In addition, a variety of functionals including BPW91 [26, 27], B3PW91 [28], and B3PLYP ([27,28]) were considered for geometry optimization of 1 in order to establish an appropriate level of theory before the more costly calculations involving 2 were undertaken. The results of the DF calculations were compared with those of the more traditional MP2 [29,30] technique. It was found that all DF and MP2 methods behaved essentially equivalently in this study, and that the accuracy of the results is more dependent on quality of basis set. Based on assessments of the results of 1, it was then considered most economical to optimize the geometry of 2 at only the BPW91/dz(d) level of theory. Frequency calculations were performed where appropriate to establish the character of minima found. The quantitative measure of the delocalization stabilization was estimated by using one of a variety of possible reaction schemes for bond separation [31,32]. We have previously found that a similar qualitative picture results over all treatments [33,34]. Additionally, NMR shielding tensors were calculated from fully optimized sturctures at select levels of theory, by using the Gauge-Independent Atomic Orbital (GIAO) method [35–37]. As with the geometry optimizations, several basis sets were used for NMR calculations on [18]annulene (1) and we found these levels to be self-consistent. Such basis sets [38,39] are necessary to describe properly the NMR properties of structures with such complex interations [40–42].
- 19 M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. Su, T. L. Windus, S. T. Elbert, J. Comput. Chem. 1993, 14, 1347.
- 20 R. Ditchfield, W. J. Hehre, J. A. Pople, J. Chem. Phys. 1971, 54, 724.
- 21 W. J. Hehre, R. Ditchfield, J. A. Pople, J. Chem. Phys. 1972, 56, 2257.
- 22
T. H. Dunning, Jr.,
P. J. Hay,
Methods of Electronic Structure Theory,
Plenum, New York,
1977, p. 1–17.
10.1007/978-1-4757-0887-5_1 Google Scholar
- 23 R. D. Amos, J. E. Rice, The Cambridge Analytical Derivatives Package Cambridge, 1995.
- 24 M. J. Frisch, G. W. Trucks, H. B. Schlegel, P. M. W. Gill, B. G. Johnson, M. A. Robb, J. R. Chesseman, T. A. Keith, G. A. Petersson, J. A. Montgomery, K. Raghavachari, M. S. Al-Laham, V. G. Zakrzewski, J. V. Ortiz, J. B. Foresman, J. Cioslowski, B. B. Stefanov, A. Nanayakkara, M. Challacombe, C. Y. Peng, P. Y. Ayala, W. Chen, M. W. Wong, J. L. Andres, E. S. Replogle, R. Gomperts, R. L. Martin, D. J. Fox, J. S. Binkley, D. J. DeFrees, J. Baker, J. P. Stewart, M. Head-Gordon, C. Gonzalez, J. A. Pople, Gaussain, Inc., Pittsburg, PA, 1994.
- 25 T. H. Dunning, Jr., P. J. Hay, Modern Theoretical Chemistry, Plenum, New York, 1976, pp. 1–28.
- 26 A. D. Becke, Phys. Rev. A 1988, 38, 3098.
- 27 J. P. Perdew, Y. Wang, Phys. Rev. B 1992, 45, 13244.
- 28 A. D. Becke, J. Chem. Phys. 1993, 98, 5648.
- 29 S. Saebo, J. Almlöf, Chem. Phys. Lett. 1989, 154, 83.
- 30 C. Møller, M. S. Plesset, Phys. Rev. 1934, 46, 618.
- 31 P. George, M. Trachman, C. W. Bock, A. M. Bret, J. Chem. Soc. Perkin Trans. 2 1976, 1222.
- 32 B. A. Hess, Jr., L. J. Schaad, J Am. Chem. Soc. 1983, 105, 7500.
- 33 K. K. Baldridge, M. S. Gordon, J. Organomet. Chem. 1984, 271, 369.
- 34 K. K. Baldridge, M. S. Gordon, J. Am. Chem. Soc. 1988, 110, 4204.
- 35 R. Ditchfield, J. Mol. Phys. 1974, 27, 789.
- 36 R. McWeeny, Phys. Rev. 1962, 126, 1028.
- 37 F. London, J. Phys. Radium (Paris) 1937, 8, 397.
- 38 A. D. McLean, G. S. Chandler, J. Chem. Phys. 1980, 72, 5639.
- 39 R. Krishnan, J. S. Binkley, R. Seeger, J. A. Pople, J. Chem. Phys. 1980, 72, 650.
- 40 T. Onak, M. Diaz, M. Barfield, J. Am. Chem. Soc. 1995, 117, 1403.
- 41 S. A. Perera, R. J. Bartlett, P. von R. Schleyer, J. Am. Chem. Soc. 1995, 117, 8476.
- 42 M. Barfield, J. Am. Chem. Soc. 1995, 117, 2862.
- 43 S. Gorter, R. Rutten-Keulemans, M. Krever, C. Romers, D. W. J. Cruick-shank, Acta Crystallogr. Sect. B 1995, 51, 1036.
- 44 H.-B. Bürgi, K. K. Baldridge, K. Hardcastle, N. L. Frank, P. Gantzel, J. S. Siegel, J. Ziller, Angew. Chem. 1995, 107, 1575; Angew. Chem. Int. Ed. Engl. 1995, 34, 1454.
- 45 S. Shaik, S. Zihlberg, Y. Hass, Acc. Chem. Res. 1996, 29, 211.
- 46 Y. Gaoni, A. Melera, F. Sondheimer, R. Wolovsky, Proc. Soc. London A 1964, 397.
- 47 Energy value in brackets includes the thermal and scaled zero-point vibrational energy corrections for 1.