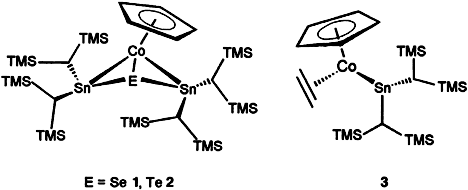
The Reactivity of the Unbridged CoSn Bond in [(η5-C5H5)(η2-C2H4)CoSn{CH[Si(CH3)3]2}2]—The First Organometallic Complexes with direct CoSnChalcogen Bonding (Chalcogen = Se, Te)†
Corresponding Author
Priv.-Doz. Dr. Jörg J. Schneider
Anorganisch-chemisches Institut der Universität, Universitätsstrasse 5–7, D-45117 Essen (Germany), Fax: Int. code + (201) 183-2402, e-mail: [email protected]
Anorganisch-chemisches Institut der Universität, Universitätsstrasse 5–7, D-45117 Essen (Germany), Fax: Int. code + (201) 183-2402, e-mail: [email protected]Search for more papers by this authorDipl.-Chem. Jörg Hagen
Anorganisch-chemisches Institut der Universität, Universitätsstrasse 5–7, D-45117 Essen (Germany), Fax: Int. code + (201) 183-2402, e-mail: [email protected]
Search for more papers by this authorDipl.-Ing. Dieter Bläser
Anorganisch-chemisches Institut der Universität, Universitätsstrasse 5–7, D-45117 Essen (Germany), Fax: Int. code + (201) 183-2402, e-mail: [email protected]
Search for more papers by this authorProf. Dr. Roland Boese
Anorganisch-chemisches Institut der Universität, Universitätsstrasse 5–7, D-45117 Essen (Germany), Fax: Int. code + (201) 183-2402, e-mail: [email protected]
Search for more papers by this authorProf. Dr. Carl Krüger
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim (Germany)
Search for more papers by this authorCorresponding Author
Priv.-Doz. Dr. Jörg J. Schneider
Anorganisch-chemisches Institut der Universität, Universitätsstrasse 5–7, D-45117 Essen (Germany), Fax: Int. code + (201) 183-2402, e-mail: [email protected]
Anorganisch-chemisches Institut der Universität, Universitätsstrasse 5–7, D-45117 Essen (Germany), Fax: Int. code + (201) 183-2402, e-mail: [email protected]Search for more papers by this authorDipl.-Chem. Jörg Hagen
Anorganisch-chemisches Institut der Universität, Universitätsstrasse 5–7, D-45117 Essen (Germany), Fax: Int. code + (201) 183-2402, e-mail: [email protected]
Search for more papers by this authorDipl.-Ing. Dieter Bläser
Anorganisch-chemisches Institut der Universität, Universitätsstrasse 5–7, D-45117 Essen (Germany), Fax: Int. code + (201) 183-2402, e-mail: [email protected]
Search for more papers by this authorProf. Dr. Roland Boese
Anorganisch-chemisches Institut der Universität, Universitätsstrasse 5–7, D-45117 Essen (Germany), Fax: Int. code + (201) 183-2402, e-mail: [email protected]
Search for more papers by this authorProf. Dr. Carl Krüger
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-45470 Mülheim (Germany)
Search for more papers by this authorJ. J. S. thanks the Deutsche Forschungsgemeinschaft (Heisenberg-Stipendium and SCHN-375/3-1) as well as the Fonds de Chemischen Industrie for support. We thank Dr. D. Stöckigt and co-workers (Max-Planck-Institut für Kohlenforschung) for recording the mass spectra.
Graphical Abstract
Butterflylike frameworks are present in the isostructural clusters 1 and 2, in which the chalcogen atom is bound as a μ3 ligand to Co and Sn. These complexes are formed by Se/Te addition to the unbridged CoSn bond of 3 at room temperature. Compounds 1 and 2 are the first defined molecular complex compounds that contain the typical semiconductor combination Sn/E (E = Se, Te) and a ferromagnetic metal, and thus they could be of interest for applications in materials science.
References
- 1(a)
D. W. Stephan,
Coord. Chem. Rev.
1989,
95, 41;
(b)
S. Friedrich,
H. Memmler,
L. H. Gade,
W.-S. Li,
M. McPartlin,
Angew. Chem.
1994,
106, 705;
Angew. Chem. Int. Ed. Engl.
1994,
33, 676;
(c)
S. Friedrich,
L. H. Gade,
I. J. Scowen,
M. McPartlin,
Angew. Chem. Int. Ed. Engl.
1996,
108, 1440 bzw.
10.1002/ange.19961081221 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1338; (d) Organometallics 1995, 14, 5344; (e) D. Selent, R. Beckhaus, R. Pickardt, Organometallics 1993, 12, 2857; (f) L. H. Gade, Angew. Chem. 1996, 108, 2225;10.1002/ange.19961081807 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 2089.
- 2(a) A. M. Glass, Science 1987, 235, 1003; (b) E. Oh, A. K. Ramdas, J. K. Furdyna, J. Lumin. 1992, 52, 183; H. Ehrenreich, Science 1987, 235, 1029; (c) M. Ilegems, R. Dingle, L. W. Rupp, Jr., J. Appl. Phys. 1975, 46, 3059; (d) S. von Molnar in Diluted Magnetic Semiconductors (Eds.: R. L. Agarwal, J. K. Furdyna, S. von Molnar), 1987, p. 39.
- 3(a) P. Boudjouk, D. J. Seidler, D. Grier, G. J. McCarthy, Chem. Mater. 1996, 8, 1189; (b) J. Arnold, J. M. Walker, K. M. Yu, P. J. Bonasia, A. L. Seligson, E. D. Bourret, J. Cryst. Growth 1992, 124, 647; (c) J. Arnold, P. J. Bonasia (University of California, Berkeley), USA 5157136, 1992; (d) A. L. Seligson, J. Arnold, J. Am. Chem. Soc. 1993, 115, 8214; (e) U. Siemeling, Angew. Chem. 1993, 105, 70; Angew. Chem. Int. Ed. Engl. 1993, 32, 67.
- 4(a) M. L. Steigerwald, Mat. Res. Soc. Symp. Proc. 1989, 131, 37; (b) K. M. Mc Gregor, G. B. Deacon, R. S. Dickson, G. D. Fallon, R. S. Rowe, B. O. West, J. Chem. Soc. Chem. Commun. 1990, 1293; (c) M. L. Steigerwald, T. Siegrist, E. M. Gyorgy, B. Hessen, Y.-U. Kwon, S. M. Tanzler, Inorg. Chem. 1994, 33, 3389.
- 5(a) D. R. Carey, J. Arnold, Inorg. Chem. 1994, 33, 1791; (b) A. R. Strezelicki, P. A. Timinski, B. A. Hebel, P. A. Bianconi, J. Am. Chem. Soc. 1992, 114, 3159; (c) M. Breuer, Khasnis, M. Buretea, M. Berardini, T. J. Emge, J. G. Brennan, Inorg. Chem. 1994, 33, 2743; (d) M. Berardini, T. Emge, J. G. Brennan, J. Am. Chem. Soc. 1994, 116, 6941; (e) D. V. Khasnis, M. Brewer, J. S. Lee, T. J. Emge, J. G. Brennan, J. Am. Chem. Soc. 1994, 116, 7129.
- 6 K. Jonas, E. Deffense, D. Habermann, Angew. Chem. 1983, 95, 729; Angew. Chem. Int. Ed. Engl. 1983, 22, 716; Angew. Chem. Suppl. 1983, 1005.
- 7 R. G. Beevor, S. A. Frith, J. L. Spencer, J. Organomet. Chem. 1981, 221, C 25.
- 8 P. J. Davidson, D. H. Harris, M. F. Lappert, J. Chem. Soc. Dalton Trans. 1976, 2268.
- 9 K. Angermund, K. Jonas, C. Krüger, J. L. Latten, Y.-H. Tsay, J. Organomet. Chem. 1988, 353, 17.
- 10
X-ray crystal structure analysis: 3a: C21H47CoSi4Sn, M = 595.64 gmol−1, T = 190 K, crystal dimensions: 0.34 × 0.27 × 0.13 mm3, R3m /V-Nicolet four-circle diffractometer (MoKα radiation, graphite monochromator), triclinic, cell dimensions (from 40 reflections, 2θ range 20–25°): a = 9.061(2), b = 11.056(3), c = 16.132(4) Å, α = 75.70(3), β = 85.86(3), γ = 67.67(3)°, V = 1447.1(3) Å3; space group P1 (no. 2), Z = 2, ρcalcd = 1.353 Mgm−3, μ = 1.685 mm−1, empiricial absorption correction with Ψ scan data, max/min transmission 1.00/0.761, Rmerg before/after correction 0.0538/0.0360, 2θmax = 45°, 5143 measured, 3770 independent, 3236 observed intensities [I > 2σ(I)] (R merg = 0.0858). Structure solution with SHELXS and refinement against F2 (SHELXTL-Plus version 5.03/Iris), 249 parameters, hydrogen atoms refined with a riding model with a common isotropic displacement factor; R1 = 0.0425 [I > 2σ(I)], wR2 = 0.0919, w−1 = σ2(F
 ) + 0.0033P, where P = [(max F
) + 0.0033P, where P = [(max F , +2F
, +2F )]/3 ist. 5: C33H81CoSeSi8Sn2, M = 1079.0 gmol−1, crystal dimensions: 0.12 × 0.41 × 0.36 mm3, a = 9.3161(2), b = 15.216(4), c = 36.6880(9) Å, V = 5200.5(14) Å3, T = 173 K, ρcalcd = 1.38 gcm−3, μ = 2.166 mm−1, Z = 4, orthorhombic, space group P212121 (no. 19), Siemens-SMART CCD system, λ = 0.71069 Å, 19195 measured reflections (±h, +k, +l), [(sinθ)/λ]max 0.83 Å−1, 19195 independent and 12680 observed reflections [I ≦ 2σ(I)], 406 refined parameters; heavy atom method, H atom positions calculated and not refined, R = 0.0629, R
)]/3 ist. 5: C33H81CoSeSi8Sn2, M = 1079.0 gmol−1, crystal dimensions: 0.12 × 0.41 × 0.36 mm3, a = 9.3161(2), b = 15.216(4), c = 36.6880(9) Å, V = 5200.5(14) Å3, T = 173 K, ρcalcd = 1.38 gcm−3, μ = 2.166 mm−1, Z = 4, orthorhombic, space group P212121 (no. 19), Siemens-SMART CCD system, λ = 0.71069 Å, 19195 measured reflections (±h, +k, +l), [(sinθ)/λ]max 0.83 Å−1, 19195 independent and 12680 observed reflections [I ≦ 2σ(I)], 406 refined parameters; heavy atom method, H atom positions calculated and not refined, R = 0.0629, R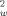 = 0.1051 [w = 1/(σ2(F
= 0.1051 [w = 1/(σ2(F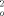 ) +(0.0395P2 +10.1396P), where P = (F
) +(0.0395P2 +10.1396P), where P = (F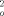 +2F
+2F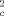 )/3], max. residual electron density 1.573 e Å−3. 6: C33H81CoSi8Sn2Te, M = 1127.64 gmol−1, T = 295 K, crystal dimensions: 0.37 × 0.24 × 0.19 mm3, Siemens-SMART-CCD area detector (MoKα radiation, graphite monochromator) orthorhombic, cell dimensions from the positions of 7479 reflections: a = 9.3582(5), b = 15.4363(9), c = 36.924(2) Å3; V = 5333.9(5) Å3; space group P212121 (no. 19), Z = 4, ρber = 1.399 Mgm−3, μ = 1.972 mm−1, empiricial absorption correction with redundant data, max./min. transmission 0.740/0.523, Rmerg before/after correction 0.0531/0.0373, θmax = 25.8° detector distance 5.891 cm, hemisphere scan in w with 0.3°, step width and three data sets of 606, 435, and 230 frames with Phi = 0,88, and 180°, in which nominally more than 97% of the data were recorded, 22934 measured, 9058 independent, 8307 observed intensities [I > 2σ(I)] (Rmerg = 0.0361); structure solution with SHELXS and refinement against F2 (SHELXTL-Plus version 5.03/Iris, 421 parameters, hydrogen atoms refined with a riding model with an isotropic displacement factor 1.2/1.5 times (for methyl groups) of that of the corresponding carbon atom; R1 = 0.0488 [I > 2σ(I)], wR2 = 0.1114, GOOF(F2) = 1.047, w−1 = σ2(F
)/3], max. residual electron density 1.573 e Å−3. 6: C33H81CoSi8Sn2Te, M = 1127.64 gmol−1, T = 295 K, crystal dimensions: 0.37 × 0.24 × 0.19 mm3, Siemens-SMART-CCD area detector (MoKα radiation, graphite monochromator) orthorhombic, cell dimensions from the positions of 7479 reflections: a = 9.3582(5), b = 15.4363(9), c = 36.924(2) Å3; V = 5333.9(5) Å3; space group P212121 (no. 19), Z = 4, ρber = 1.399 Mgm−3, μ = 1.972 mm−1, empiricial absorption correction with redundant data, max./min. transmission 0.740/0.523, Rmerg before/after correction 0.0531/0.0373, θmax = 25.8° detector distance 5.891 cm, hemisphere scan in w with 0.3°, step width and three data sets of 606, 435, and 230 frames with Phi = 0,88, and 180°, in which nominally more than 97% of the data were recorded, 22934 measured, 9058 independent, 8307 observed intensities [I > 2σ(I)] (Rmerg = 0.0361); structure solution with SHELXS and refinement against F2 (SHELXTL-Plus version 5.03/Iris, 421 parameters, hydrogen atoms refined with a riding model with an isotropic displacement factor 1.2/1.5 times (for methyl groups) of that of the corresponding carbon atom; R1 = 0.0488 [I > 2σ(I)], wR2 = 0.1114, GOOF(F2) = 1.047, w−1 = σ2(F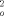 ) +(0.0531P)2 +18.47P, where P = [(max F
) +(0.0531P)2 +18.47P, where P = [(max F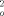 ,O) + 2F
,O) + 2F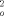 )]/3, absolute structure parameter–0.01(3). The anisotropic displacement factors of several methyl groups, particularly at C25, indicate disorder, which, however, cannot be resolved. Further details of the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, D-76344 Eggenstein- Leopoldshafen (Germany), on quoting the depository numbers CSD-100065, CSD- 406237, and CSD-406 281.
)]/3, absolute structure parameter–0.01(3). The anisotropic displacement factors of several methyl groups, particularly at C25, indicate disorder, which, however, cannot be resolved. Further details of the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, D-76344 Eggenstein- Leopoldshafen (Germany), on quoting the depository numbers CSD-100065, CSD- 406237, and CSD-406 281.
- 11(a) C. J. Cardin, D. J. Cardin, G. A. Lawless, J. M. Power, M. B. Hursthouse, J. Organomet. Chem. 1987, 325, 203; (b) C. J. Cardin, D. J. Cardin, H. E. Parge, J. M. Power, J. Chem. Soc. Chem. Commun. 1984, 609; (c) C. J. Cardin, D. J. Cardin, J. M. Power, J. Am. Chem. Soc. 1985, 107, 505; (d) S. M. Hawkins, P. B. Hitchcock, M. F. Lappert, J. Chem. Soc. Chem. Commun. 1985, 1592.
- 12 The NiSn distance in [η2-C2H4)2NiSn{CH(SiMe3)2}2] of 2.387(1) Å. which is similar to that in 3a, is indicative of NiSn bonding: C. Pluta, K. R. Pörschke, R. Mynott, P. Betz, C. Krüger, Chem. Ber. 1991, 124, 1321.
- 13 We have recently prepared the Ni/Sn complex analogous to 4: J. J. Schneider, J. Hagen, unpublished results.
- 14 R. A. Zingaro, B. H. Stevens, K. Irgolic, J. Organomet. Chem. 1965, 4, 320.
- 15 J. J. Schneider, J. Hagen, O. Heinemann, J. Bruckmann, C. Krüger, Thin Film Solids 1997, in press.