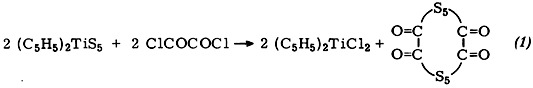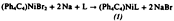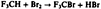Journal list menu
Export Citations
Download PDFs
Graphical Abstract (Angew. Chem. Int. Ed. Engl. 2/1980)
- First Published: February 1980
Reviews
Raw Material-Polymer Interrelationships — Today and Tomorrow†‡
- Pages: 79-87
- First Published: February 1980
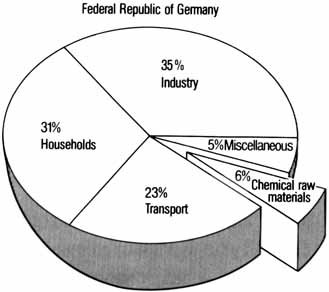
Petroleum, natural gas, and coal are presently employed not only as raw materials but primarily as sources of energy. One of the most pressing needs is to disengage this double function and to better utilize fossil resources. Possible solutions are demonstrated by means of plastics (a breakdown of oil consumption in the Federal Republic of Germany in 1976 is shown on the right).
Chemistry and Function of Human Plasma Proteins†
- Pages: 87-99
- First Published: February 1980
Components of the blood clotting system, immunoglobulins, lipoproteins, and numerous other proteins—numbering over 100—have been isolated from blood plasma by new techniques. Some of the biologically active proteins obtainable in this way possess considerable prophylactic and therapeutic potential. In fact, blood plasma would appear too valuable for mere transfusion.
New Technologies for the Filmless Manufacture of Printing Forms†‡
- Pages: 99-110
- First Published: February 1980
The innovative pressure exerted by electronics on the processing of optical information poses the chemist new tasks: apart from the chemical properties of materials, their electrical properties are becoming increasingly important. Chemicals suitable for production of reprographic forms by electrophotography without film include CdS, ZnO, and organic heterocycles such as oxazoles and benzothiazoles.
On the Symbolic Language of the Chemist†
- Pages: 110-125
- First Published: February 1980
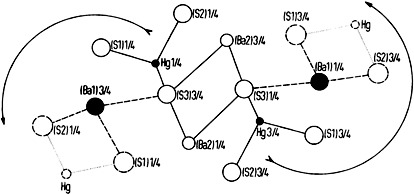
In a stoke of genius, Berzelius created the formula language of chemistry over 100 years ago. This language has hardly changed since then. However, it is just not informatic enough to deal with inorganic solid state compounds, and more recent proposals also appear rather inadequate. The article closes with a call for a new Berzelius.
Communications
Synthesis and Crystal Structure Analysis of Decathiacyclotetradecane-6,7,13,14-tetraone, S10(CO)4†‡
- Pages: 125-126
- First Published: February 1980
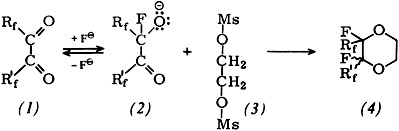
1,4-Dioxanes with Four Fluorine and/or Trifluoromethyl Substituents on C2 and C3 from Vicinal Perfluorodicarbonyl Compounds†‡
- Page: 126
- First Published: February 1980
A Selective Pathway from Methanol to Ethylene and Propene†‡
- Pages: 126-127
- First Published: February 1980
Assured supplies of raw materials is of topical interest in view of the threatening scarcity of petroleum. Mu-doped aluminosilicate catalysts transform methanol into a surprisingly narrow range of products containing a large proportion of ethylene and propene. Methanol is accessible, in principle, from all carbonaceous materials via synthesis gas.
Addition of Alkanethiols to Diketene†
- Pages: 127-128
- First Published: February 1980
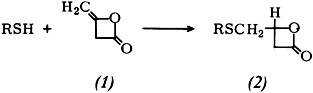
Reactions of RSH with diketene (1) are highly dependent upon the reaction conditions. Acid-catalyzed reaction of alkanethiols with (1) leads, like the photo or radical reaction, to the lactone (2). This could not be deduced from previous findings. (2) reacts with alcohols to give γ-(alkylthio)crotonic esters.
Direct Synthesis of the Perfluoroalkanesulfinates of Iron(II), Cobalt(II), and Nickel(II): SO2-Assisted Addition of Alkyl Halides to Transition Metals†
- Pages: 128-129
- First Published: February 1980
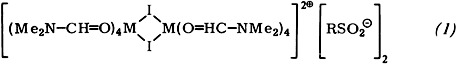
Reactions of typical transition metals with alkyl halides normally take place only after prior chemical or physical activation of the metals. It is now possible to bring about reaction of commercial grade Fe, Co, and Ni powder with perfluoroalkyl iodides (RI) under mild conditions: In the presence of SO2, the alkanesulfinates (1) with a dinuclear cation are formed in dimethylformamide at room temperature.
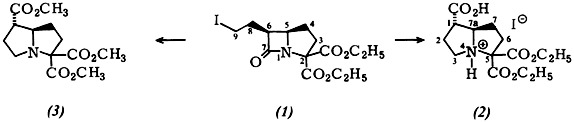
Pyrrolizidines by Rearrangement of β-Lactams†
- Pages: 129-130
- First Published: February 1980
Syntheses with Cyanogen—A Simple Route to 4,5-Dichloroimidazoles†
- Pages: 130-131
- First Published: February 1980
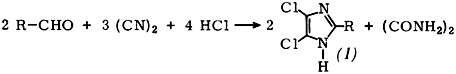
4,5-Dichlorinated imidazoles (1) having an unsubstituted N atom were not previously accessible by a general synthesis. It has now proved possible to produce numerous derivatives (1) in useful yields by synthesis from aldehydes, cyanogen, and hydrogen chloride, thus permitting a study of their reactions.
Synthesis and Structure of 3-Chloro-4-hydroxy- and 3,4-epoxyphospholane 1-Oxides†
- Pages: 132-133
- First Published: February 1980
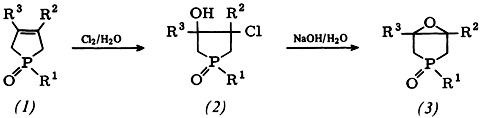
Epoxidation of phospholene oxides (1) to give (2) proceeds almost quantitatively by the route shown below, thus superseding previous methods. There is no need to isolate the intermediate (2). An X-ray structure analysis has been performed on one of the stereoisomers of (2a), R1 = OCH3, R2 = R3 = CH3.
New Peptide Synthesis†
- Pages: 133-134
- First Published: February 1980
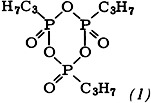
Alkylphosphonic anhydrides such as (1) are recommended as condensation reagents for peptide synthesis. One advantage of (1) over dicyclohexylcarbodiimide (DCC) is that no sparingly soluble secondary products are formed. With regard to yield and degree of racemization, the new method is just as good as improved DCC techniques, and in some cases superior.
Stereocontrolled Intramolecular Diels-Alder Reaction of Heterodienes; Studies on the Synthesis of Cannabinoids†
- Pages: 134-135
- First Published: February 1980
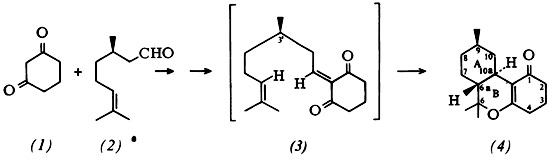
The skeleton (4) of tetrahydrocannabinol having translinked rings A and B can be obtained optically pure from the diketone (1) and (R)-citronellal (2). The key step is intramolecular Diels-Alder reaction (up to 20°C!) of the heterodiene intermediate (3).—Derivatives of (4) are of interest, e.g., in the treatment of glaucoma.
(3R,S)-3,4-Dicarboxy-3-hydroxybutyl Coenzyme A, an Inhibitor of the Citrate Synthase Reaction
- Page: 136
- First Published: February 1980
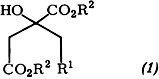
Citrate synthase, an enzyme of the tricarboxylic acid cycle, catalyzes the formation of citrate and coenzyme A from acetyl CoA and oxalacetate. In order to establish whether citryl CoA is formed as a true intermediate on the synthase, the analogue (1), R1 = CH2SCoA, R2 = H, has been synthesized (citryl CoA contains CO in place of CH2). Kinetic measurements showed that (1) acts as a strong inhibitor.
Tetrakis(phenylimino)cyclobutane (Tetrameric Phenyl Isocyanide)†
- Pages: 136-137
- First Published: February 1980
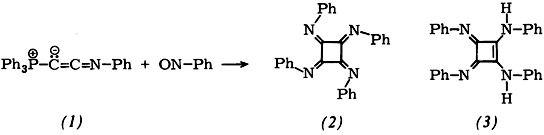
A nitrogen analogue of the hitherto unknown cyclobutane tetraone, the title compound (2), is formed as red-violet crystals from the phosphorane (1) and nitrobenzene in a one-pot reaction. The arrangement of phenyl groups indicated in the formula is confirmed by X-ray structure analysis. (2) is reduced, e.g. by phenylhydrazine, to (3), the first amidine of squaric acid whose N atoms are not linked in a ring.
Tetrachlorovinylcarbene from Tetrachlorocyclopropene; Facile Synthesis of Vinylcyclopropanes†‡
- Pages: 138-140
- First Published: February 1980
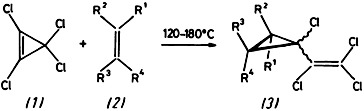
Tetrachlorovinylcarbene, ClC̈CClCCl2, is unexpectedly formed on reaction of tetrachlorocyclopropene (1) with alkenes (2), in which the adducts (3) can be isolated. As shown by numerous examples, this type of reaction can be generalized, e.g. for synthesis of acids and esters (3), R1 = CO2H and CO2R, having potential as insecticides.
Mutual Interconversion of the Chromophoric Systems of Porphyrinogen and Isobacteriochlorin†
- Pages: 140-141
- First Published: February 1980
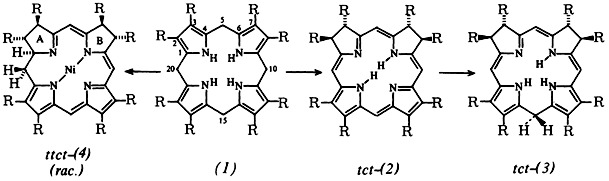
Reactions of hexahydroporphyrins in the absence of air are strongly influenced by metal ions. Thus the tetrapyrrolic porphyrinogen (1), R = C2H5, reacts in the presence of Co2+ via several steps, including isobacteriochlorins (2), to give the hemicorrinoid dipyrrolic compounds (3). These are tautomers of (1). In the presence of Ni2+, (1) form complexes of the new hemicorrinoid-pyrromethenic system (4). ttct-(4) and tttt-(4) were characterized by their spectra and also by X-ray structure analysis. (The species ttt-(2), ttt-(3), and tttt-(4) likewise formed are not included in the scheme below.)
1,2,3,7,8,20-Hexahydroporphyrin, an Easily Formed Ligand System Isomeric to Porphyrinogens†
- Pages: 141-143
- First Published: February 1980
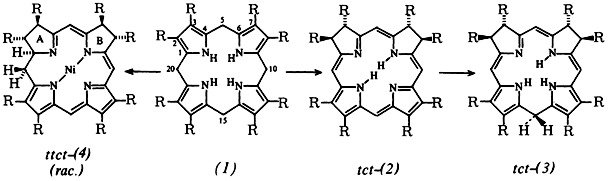
Reactions of hexahydroporphyrins in the absence of air are strongly influenced by metal ions. Thus the tetrapyrrolic porphyrinogen (1), R = C2H5, reacts in the presence of Co2+ via several steps, including isobacteriochlorins (2), to give the hemicorrinoid dipyrrolic compounds (3). These are tautomers of (1). In the presence of Ni2+, (1) form complexes of the new hemicorrinoid-pyrromethenic system (4). ttct-(4) and tttt-(4) were characterized by their spectra and also by X-ray structure analysis. (The species ttt-(2), ttt-(3), and tttt-(4) likewise formed are not included in the scheme below.)
Synthesis and Transformations of 5-Cyano-2,2,8,8,12,13,17,18-octamethylisobacteriochlorin†
- Pages: 143-145
- First Published: February 1980

A model substrate for studies in the hexahydroporphyrin series, i.e. the title compound (1) having C2v symmetry, has been prepared by a multistep de-novo synthesis from a cyanosubstituted thiolactam. Catalytic hydrogenation of (1) affords the highly O2-sensitive 2,3,7,8,15,24-hexahydro derivative; attempts to incorporate metal ions into this species lead to complexes of the 1,2,3,7,8,20-hexahydro isomer.
Tetraphenylcyclobutadienecycloalkenenickel(0) Complexes
- Pages: 145-146
- First Published: February 1980
Nickel(0) complexes having only one tetraphenylcyclobutadiene ligand (Ph4C4) and one cycloalkene ligand (L) are accessible with surprising ease, e.g. by prolonged stirring of the reagents given below in either at −50°C. (1a) and (1b), green crystals, only decompose at 220 and 260°C, respectively. [(1a), L = 1,5-cyclooctadiene; (1b), L = cyclooctatetraene.]
Photochemically Induced 1,2-Bis(dialkylalumination) of Diphenylacetylene
- Page: 146
- First Published: February 1980
Heterogeneously Catalyzed Gas-Phase Bromination of Trifluoromethane†‡
- Pages: 147-148
- First Published: February 1980
The optimization of a heterogeneously catalyzed gas-phase reaction by PE spectroscopy is demonstrated for the production of F3CBr, an extinguishing agent used in automatic fire extinguishing installations. A specially developed (PE spectroscopic) gas-analysis technique permits screening of catalysts (and conditions). The best results were obtained with FeCl3/KBr/activated charcoal.
Book Reviews
Book Review: Ullmanns Encyklopädie der technischen Chemie [Ullmann's Encyclopedia of Industrial Chemistry]. Editorial board E. Bartholomé, E. Biekert, H. Hellman, H. Ley (deceased), W. M. Weigert (deceased), and E. Weise
- Page: 148
- First Published: February 1980
Book Review: The Chemical Applications of Transmission Electron Microscopy. J. R. Fryer
- Page: 148
- First Published: February 1980
Book Review: Treatise on Analytical Chemistry. Part I: Theory and Practice. Edited by I. M. Kolthoff et al.
- Pages: 149-150
- First Published: February 1980