Stereocontrolled Intramolecular Diels-Alder Reaction of Heterodienes; Studies on the Synthesis of Cannabinoids†
This work was supported by the Fonds der Chemischen Industrie. We wish to thank Prof. Dr. D. Leibfritz and Dr. M. Feigl, Universität Bremen, for recording the 360-MHz 1H-NMR spectrum and the Dragoco Company, Holzminden, for supplying citronellal.
Graphical Abstract
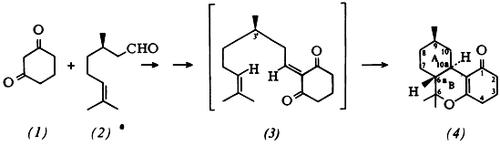
The skeleton (4) of tetrahydrocannabinol having translinked rings A and B can be obtained optically pure from the diketone (1) and (R)-citronellal (2). The key step is intramolecular Diels-Alder reaction (up to 20°C!) of the heterodiene intermediate (3).—Derivatives of (4) are of interest, e.g., in the treatment of glaucoma.