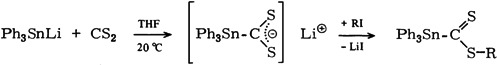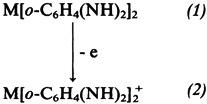Journal list menu
Export Citations
Download PDFs
Graphical Abstract (Angew. Chem. Int. Ed. Engl. 3/1980)
- First Published: March 1980
Reviews
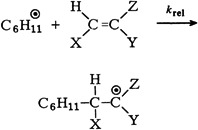
Electrophilic Reagents—Recent Developments and Their Preparative Application†
- Pages: 151-171
- First Published: March 1980
Coupling of Solvolysis and CC Linkage: A Promising Synthetic Route to Functionalized Carboxylic Acids, Aldehydes, and Ketones†
- Pages: 171-178
- First Published: March 1980
Synthesis of Intermediates by Rhodium-Catalyzed Hydroformylation†
- Pages: 178-183
- First Published: March 1980
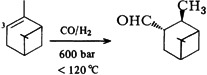
“Oxo technology”—i.e. reactions of olefins with CO and H2 to give aldehydes and their reaction products—profits from the use of Rh catalysts, which are 103 to 104-fold more effective than the conventional Co catalysts. Thus pressure and temperature can be varied more widely and the selectivity greatly enhanced, reaching 85% in the example shown.
3-Phenylpropylamines, A New Class of Systemic Fungicides†
- Pages: 184-189
- First Published: March 1980
Developments in the Field of Inorganic Pigments†‡
- Pages: 190-196
- First Published: March 1980
The interplay of solid-state properties, particle size, and particle shape determines the specific properties of inorganic pigments. The possible uses of the pigments also depend upon the phenomena occurring at their interfaces. Advances have been made primarily in the fields of transparent pigments and magnetic pigments.
Communications
Ultratrace Analysis: Determination of Cr, Fe, and Co in the ppb and ppt Range for Checking the Preparation of High-Purity Niobium†
- Pages: 197-198
- First Published: March 1980
The purest niobium to be obtained so far was produced in a three-step process from commercial pure niobium and characterized by n-activation analysis. In order to measure the Cr, Fe, and Co content, carrier elements were added to the sample after irradiation for 42 d and surface decontamination. After chemical separation, the three elements could be determined by gamma-ray spectrometry (detection limits 10 ppt, 1.5 ppb, and 4.0 ppt, respectively). Concentration ranges in the five samples: Cr 10—24.7 ppt, Fe 2—6.6 ppb, Co 4—19.0 ppt.
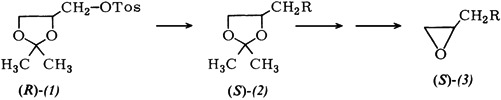
Synthesis of Monosubstituted (S)-Oxiranes in High Optical Purity†
- Pages: 198-199
- First Published: March 1980
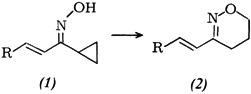
Rearrangement of Cyclopropyl Ketone Oximes to 5,6-Dihydro-4H-1,2-oxazines†
- Page: 199
- First Published: March 1980
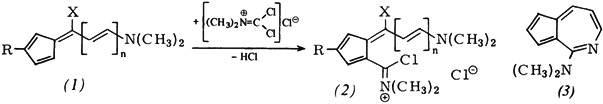
Synthesis of Carbocyclic and Heterocyclic π-Electron Systems with Pentafulvenoid Chloroformamidinium Chlorides†
- Pages: 199-201
- First Published: March 1980
Synthesis of α-Haloalkylcarbamoyl Halides†
- Pages: 201-202
- First Published: March 1980
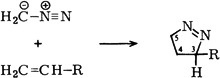
Cycloadditions of Diazoalkanes to 1-Alkenes†
- Pages: 202-203
- First Published: March 1980
Synthesis of a Homologous Tetrathiafulvalene with Central Bicyclo[4.4.1]undeca-1(10),3,5,8-tetraene-2,7-diylidene Group†‡
- Pages: 204-205
- First Published: March 1980
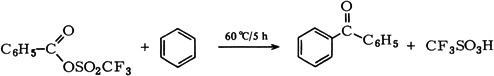
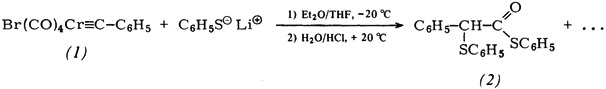
Double Addition of a Nucleophile to Carbyne and Carbonyl C-Atom: S-Phenyl 2-Phenyl-2-(phenylthio)thioacetate from trans-Bromotetracarbonyl(phenylcarbyne)chromium and Thiophenolate†
- Pages: 205-206
- First Published: March 1980
Correlation between Radical Reactivities and Copolymerization Parameters†‡
- Pages: 206-207
- First Published: March 1980
Aza[14]annulenes†‡
- Pages: 207-208
- First Published: March 1980
![Aza[14]annulenes](/cms/asset/d8c0954e-7033-456a-a88d-3b348efe32a3/must001.jpg)
An aromatic vinylog of pyridine, aza[14]annulene (1), has been obtained in a multistep synthesis from the tetracyclic species (2), R = H. The conformationally mobile (1) exists in two inseparable forms in solution (ratio 4:1). As in the longer-known aza[18]annulene, the nitrogen occupies an internal position in the major component.
1H-NMR Investigations on the Mutarotation of N-Acetyl-D-neuraminic Acid†‡
- Pages: 208-209
- First Published: March 1980
Enzymatic liberation of N-acetyl-α-D-neuraminic acid in an NMR spectrometer permitted the first determination of the mutarotation of this biochemically important sugar derivative which was formerly considered to be too fast to measure. Until recently it was assumed that only the β-form is present in aqueous solution.
Structural Changes on Oxidation: Flattening of the C4-Cluster of Tetrahedrane†
- Pages: 209-210
- First Published: March 1980
As a cluster with a “balanced” number of electrons, tetra-tert-butyltetrahedrane surprisingly affords the same radical cation as tetra-tert-butylcyclobutadiene on electron removal. The spontaneous valence isomerization can be explained on the basis of the calculated charge distribution.
Structure of Tetra-tert-butylcyclobutadiene†‡
- Pages: 211-212
- First Published: March 1980
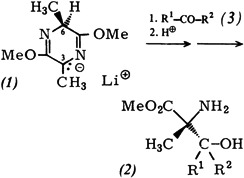
Enantioselective Synthesis of α-Methylserines†‡
- Pages: 212-213
- First Published: March 1980
Lithium [Bis(trimethylsilyl)methylene]diphenylphosphoranide, a Building Block for the Synthesis of Bis(methylenephosphoranes)†
- Pages: 213-214
- First Published: March 1980
“α,α,α”-, “α,β,α”- and “α,α,β”-Trioxatris-σ-homotropone†‡
- Pages: 214-215
- First Published: March 1980
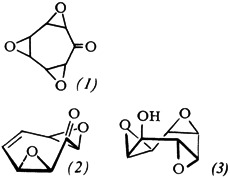
The three stereoisomeric triepoxycycloheptanones (1), which could be transformed, inter alia, into trioxonines and into potentially “tris-homobenzenoid” carbenium ions, have been synthesized separately: the two “symmetrical” isomers from readily accessible (2), and the unsymmetrical isomer from (3).
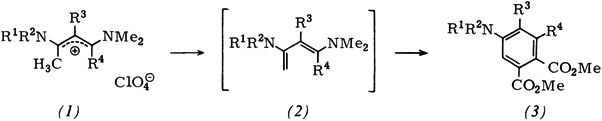
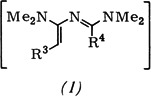
1,3-Bis(diaklyamino)-1,3-butadience from 1-Methylvinamidinium Salts†‡
- Pages: 216-217
- First Published: March 1980
1,3-Bis(dimethylamino)-2-aza-1,3-butadienes from 1-Alkyl-2-azavinamidinium Salts†‡
- Page: 217
- First Published: March 1980
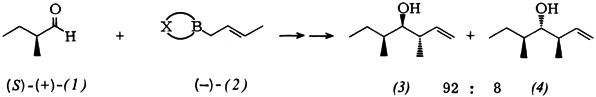
Enhancement of 1,2-Asymmetric Induction on CC Linkage by Use of Chiral 2-Butenylboronic Esters†‡
- Pages: 218-219
- First Published: March 1980
1,3-Bis(1-lithio-3-methyl-1-phenylpentyl)benzene, an Organodilithium Compound Soluble in Aromatic Hydrocarbons†‡
- Pages: 219-220
- First Published: March 1980
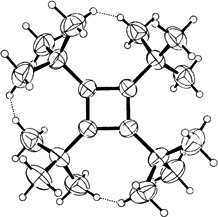
(AsPh4)2[W2NCl10], a μ-Nitrido Complex Containing Tungsten(V) and Tungsten(VI)
- Page: 220
- First Published: March 1980
Nucleophilic Addition of Triphenylstannyllithium to Carbon Disulfide
- Pages: 220-221
- First Published: March 1980
Photooxidation of the Ligands of Bis(o-semiquinonediimine)nickel(II) and -platinum(II)†
- Pages: 221-222
- First Published: March 1980
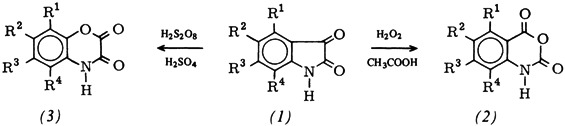
Oxidation of Isatins to Isatoic Anhydrides and 2,3-Dioxo-1,4-benzoxazines†
- Pages: 222-223
- First Published: March 1980
Influence of Isomerism of Alkanes on the Thermodynamic Properties of Cyclohexane/Alkane Mixtures†
- Pages: 223-225
- First Published: March 1980
The differing thermodynamic behavior of straight-chain and branched hydrocarbons in mixtures with other compounds is particularly important in separation processes. An extension of the Flory theory now permits a consistent description of the excess quantitites for mixtures of isomeric alkanes with cyclohexane and prediction of these quantities for regions of pressure and temperature not yet examined experimentally.
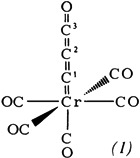
Complex Stabilization of 3-Oxopropadienylidene (C3O) with Pentacarbonylchromium(0)†
- Pages: 225-226
- First Published: March 1980
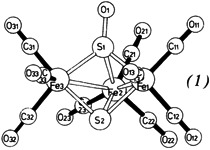
First Fixation of the Unstable Species Sulfur Monoxide in a Cluster: Synthesis and Structure of Fe3(CO)9(S)SO
- Pages: 226-227
- First Published: March 1980
Direct Photolysis of Uranium Hexafluoride as a Preparatively Utilizable Endothermic Reaction†
- Pages: 227-228
- First Published: March 1980
Laser isotopic separation of uranium could profit from UF6 photodissociation. Direct cleavage of UF6 (50 g) by UV light into UF5 and highly pure fluorine was achieved almost quantitatively (39 h) in a new apparatus. It should be possible in principle to accomplish laser isotope separation without scavengers and to recover fluorine in the nuclear fuel cycle.
Book Reviews
Book Review: Kirk-Othmer: Encyclopedia of Chemical Technology. Editorial board: H. F. Mark, D. F. Othmer, C. G. Overberger, and G. T. Seaborg
- Pages: 228-229
- First Published: March 1980
Book Review: Synthetische Arzneimittel (Synthetic Drugs). S. Ebel
- Page: 229
- First Published: March 1980
Book Review: Molecular and Crystal Structure Models. A. Walton
- Page: 229
- First Published: March 1980
Book Review: Radicals. D. C. Nonhebel, J. M. Tedder, and J. C. Walton
- Page: 229
- First Published: March 1980
Book Review: Organofluorine Chemicals and Their Industrial Applications. Edited by R. E. Banks
- Page: 230
- First Published: March 1980
Book Review: The Chemistry of Silica. Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry. R. K. Iler
- Page: 230
- First Published: March 1980





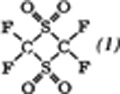
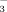 or FSO
or FSO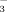 .
. 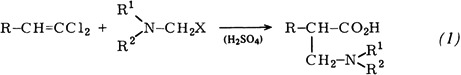
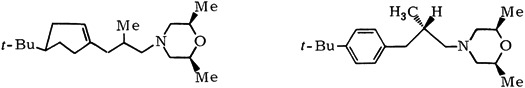
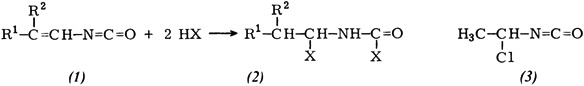
![Synthesis of a Homologous Tetrathiafulvalene with Central Bicyclo[4.4.1]undeca-1(10),3,5,8-tetraene-2,7-diylidene Group](/cms/asset/2cbc04c6-4035-4fdb-975a-64750df30ae8/must001.jpg)
![Lithium [Bis(trimethylsilyl)methylene]diphenylphosphoranide, a Building Block for the Synthesis of Bis(methylenephosphoranes)](/cms/asset/60c71485-d933-41a0-8d17-631ce4362be3/must001.jpg)
![(AsPh4)2[W2NCl10], a μ-Nitrido Complex Containing Tungsten(V) and Tungsten(VI)](/cms/asset/e84d288e-1dd7-4f95-ac2f-79e6f33d66b0/must001.jpg)