Enhancement of 1,2-Asymmetric Induction on CC Linkage by Use of Chiral 2-Butenylboronic Esters†‡
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Dedicated to Professor Matthias Seefelder on the occasion of his 60th birthday



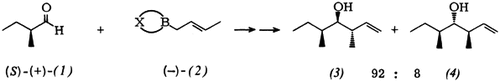
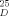 = - 5.76° (neat)) showed a rotation of [α]
= - 5.76° (neat)) showed a rotation of [α]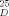 = + 32.5° (neat) (34.5° [9a]). Reduction with BH3/THF [9c] regenerated the enantiomerically pure alcohol.
= + 32.5° (neat) (34.5° [9a]). Reduction with BH3/THF [9c] regenerated the enantiomerically pure alcohol.

