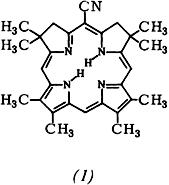
Synthesis and Transformations of 5-Cyano-2,2,8,8,12,13,17,18-octamethylisobacteriochlorin†
The Chemistry of Hexahydroporphyrins, Part 3. This work was supported by the Swiss National Science Foundation. P. N. (postdoctoral fellow at the ETH 1977/1978) gratefully acknowledges a scholarship from the German Science Foundation.—Part 2: [1b].
Graphical Abstract
A model substrate for studies in the hexahydroporphyrin series, i.e. the title compound (1) having C2v symmetry, has been prepared by a multistep de-novo synthesis from a cyanosubstituted thiolactam. Catalytic hydrogenation of (1) affords the highly O2-sensitive 2,3,7,8,15,24-hexahydro derivative; attempts to incorporate metal ions into this species lead to complexes of the 1,2,3,7,8,20-hexahydro isomer.