Journal list menu
Export Citations
Download PDFs
Cover Picture (Angew. Chem. Int. Ed. Engl. 23/24/1996)
- First Published: December 1996
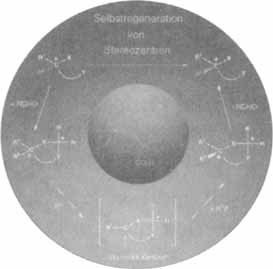
The cover picture shows the principle of the “Self-Regeneration of Stereocenters (SRS)”, a synthetic method in which a stereogenic center is temporarily destroyed with formation of an intermediate having a trigonal center. A subsequent diastereoselective reaction results in the formation of the stereogenic center once more. The additional center of chirality essential for the diastereoselective reaction is generated by acetalization (with an aldehyde) of the functional groups X and Y in the chiral starting material. The reaction sequence leads to a dissociative, enantioselecitve substitution at a single stereogenic center in a chiral compound without the use of a chiral auxiliary—hence the name “self-regeneration”.
The general formula of typical glycine derivatives is shown in yellow in the center of the scheme. These are easily prepared in enantiomerically pure form by resolution of the appropriate racemate and have been shown to be particularly useful for the synthesis of α,α-disubstituted amino acids. As these compounds are prepared from chiral starting materials, they also represent the abandonment of the synthetic principle. D. Seebach et al. report more on SRS on p. 2708 ff.
Graphical Abstract (Angew. Chem. Int. Ed. Engl. 23/24/1996)
- Pages: 2691-2701
- First Published: December 1996
Reviews
Self-Regeneration of Stereocenters (SRS)—Applications, Limitations, and Abandonment of a Synthetic Principle†‡
- Pages: 2708-2748
- First Published: December 1996
The formation of enantiopure compounds from an achiral starting material has to be one of the greatest challenges in organic synthesis today. One approach has its roots in investigations into the “Self-Regeneration of Stereocenters”. This principle was first realized with compounds containing a stereogenic center (for example the naturally occurring, enantiomerically pure amino acids), but more recently the knowledge gained in this area has been applied to achiral compounds such as glycine, 3-aminopropionic acid, and acetoacetic acid. The resulting procedures have found wide-ranging use in the synthesis of non-proteinogenic and radiolabeled amino acids, and have become particulary valuable in the synthesis of natural products with tertiary C atoms.
Erich Hückel, Pioneer of Organic Quantum Chemistry: Reflections on Theory and Experiment
- Pages: 2750-2764
- First Published: December 1996
The theoretical basis for much of the subsequent application of molecular orbital concepts to important problems of organic chemistry was derived from Erich Hückel's treatment of unsaturated and conjugated compounds in the 1930s. Yet circumstances still not fully understood delayed the recognition that he deserved, and not incidentally, retarded the development of organic chemistry by muffling the impact of his contributions.
Highlights
New Carbocations—From Physical Organic Chemistry to Biochemistry†
- Pages: 2765-2766
- First Published: December 1996
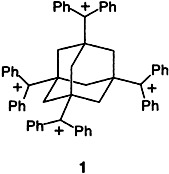
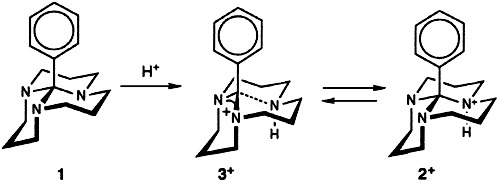
In the first carbotetracation, 1, the charged groups are tetrahedrally arranged on an adamantane cage. Carbocations are no longer a domain of physical organic chemistry. They also play a role in organometallic chemistry and biochemistry, as a new samarocene complex containing a norbornadienyl cation fragment and the newly coined term “Olah enzyme” testify.
The β Turn as a Selectivity Switch: βI or βII′? That Is the Question†
- Pages: 2767-2769
- First Published: December 1996
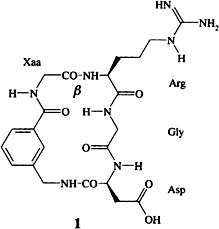
In the design of peptide-based drugs the β turn has been promoted to a design principle in recent years. In research on antiadhesive RGD mimetics, receptor selectivity has been shown to be encoded in the β-turn type within cyclic peptides of type 1 having an identical lead sequence. Switching the turn type from βII′ to βI inverts the selectivity profile so that a compound originally with antithrombotic activity becomes an antimetastatic agent.
Communications
Unprecedented Conversion of a Compound with Metal–Metal Bonding into a Solvated Molecular Wire†
- Pages: 2772-2774
- First Published: December 1996
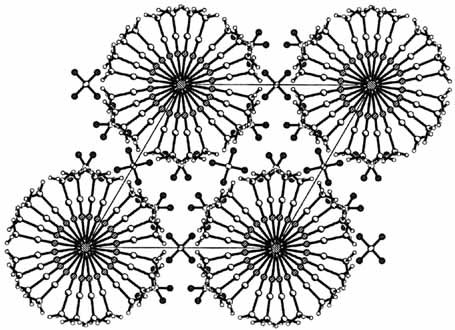
One-electron reduction of the rhodium complex 1 has resulted in the first successful conversion of a discrete metal–metal bonded “dimer” into a one-dimensional polymer (2). The doped material 2 (depicted on the right) differs significantly from the well-known tetracyanoplatinates in that the metal backbone is cationic. In addition, there is the possibility of fine-tuning the material properties by varying the organic group.
E/Z Isomerization in Crystals—Phase Rebuilding on Photolysis with Long-Wavelength Radiation†
- Pages: 2774-2777
- First Published: December 1996
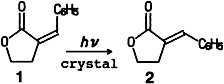
Three solid-state techniques—atomic force microscopy, X-ray structure analysis, and diffuse reflectance spectroscopy—were combined to discover why E/Z isomerizations in the crystal, for example 1 → 2, are possible despite their large spatial demands. These studies revealed, for instance, that on irradiation with wavelengths corresponding to the absorption tail of the crystals, anisotropic surface features appear.
Designed Restructuring of Iodine with Microporous SiO2†
- Pages: 2777-2779
- First Published: December 1996

Templates for the arrangement of guest molecules—that is the role played by microporous crystalline SiO2 modifications in the insertion compounds presented. The electronic interactions between the guest species can be controlled by the dimensionalities of the void systems. When iodine is the guest component, this is directly apparent from the colors of the insertion compounds, which for the host structures shown below range from violet to red-brown.
A Noninterpenetrated Molecular Ladder with Hydrophobic Cavities†
- Pages: 2779-2782
- First Published: December 1996
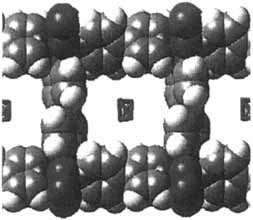
Not interpenetrated, but filled with solvent molecules, the large cavities of the polymer [Co(NO3)2(4,4′-bpy)1.5]n form different structures depending on the solvent system from which it crystallizes. One of the two possible structures (section of structure shown on the right) exhibits hydrophobic cavities about 11 × 11 Å in size. The solvent molecules can be readily removed, but the process has not yet been proven reversible.
Anion Binding: Self-Assembly of Polypyrrolic Macrocycles†‡
- Pages: 2782-2785
- First Published: December 1996
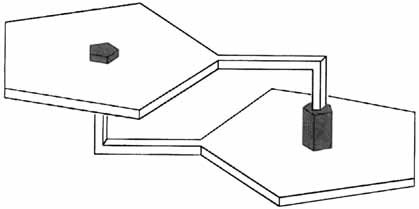
Anion complexation drives the self-assembly of appropriately designed macrocycles (sapphyrin and/or calix[4]pyrrole) containing pendant carboxylic arms (black in the schematic representation on the right). The self-assembly process can be disrupted by addition of F− ions (sapphyrin) or in the case of calix[4]pyrrole, polar solvents.
In Situ Observation of Transient Reaction Phenomena Occurring on Zeolite Catalysts with the Aid of Positron Emission Profiling
- Pages: 2785-2787
- First Published: December 1996
The total concentrations of reactants and products within a flow-through reactor can be monitored as a function of both position and time by detecting gamma photons arising from the radioactive positron decay of injected 11C nuclei. The method was demonstrated by directly observing catalyst preconditioning and deactivation of a Pt/H-mordenite catalyst bed during the industrially important hexane hydroisomerization reaction.
The Titanium(IV)-Catalyzed Epoxidation of Alkenes by tert-Alkyl Hydroperoxides†
- Pages: 2787-2790
- First Published: December 1996
A high catalytic activity towards alkene epoxidation is shown by titanium-modified MCM41 mesoporous silicas if 2-methyl-1-phenyl-2-propyl hydroperoxide (MPPH) is used as oxidant. This first example in which MPPH has been used as an efficient two-electron oxidizing agent demonstrates that it is a competent replacement for tert-butyl hydroperoxide (TBHP) in metal-catalyzed, nonradical hydrocarbon oxidations and that the Ti-MCM41 catalyzed epoxidation of alkenes proceeds by a nonradical mechanism.
Amide Backbones with Conformationally Restricted Furanose Rings: Highly Improved Affinity of the Modified Oligonucleotides for Their RNA Complements†
- Pages: 2790-2794
- First Published: December 1996
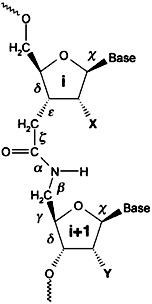
An increased stability of duplexes between amide-modified oligonucleotides (depicted on the right) and RNA was achieved by the introduction of 2′-OMe groups. Since the modified oligonucleotides display a very high affinity for their RNA complement and are more stable towards nucleases, they can be considered as promising antisense agents. X = H, OH, OMe; Y = H, OMe.
Synthesis and Enzymatic Degradation of Dendrimers from (R)-3-Hydroxybutanoic Acid and Trimesic Acid†
- Pages: 2795-2797
- First Published: December 1996
Synthesis and a Preliminary DNA Binding Study of Hybrids of the Carbohydrate Domain of Calicheamicin γ and the Aglycone of Daunorubicin: Calichearubicins A and B†
and the Aglycone of Daunorubicin: Calichearubicins A and B†
- Pages: 2797-2801
- First Published: December 1996
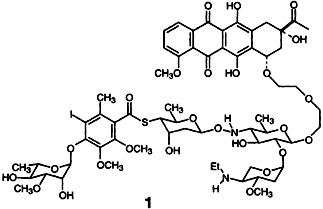
In calichearubicin B (1) the aglycon of daunorubicin and the carbohydrate domain of calicheamicin γ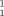 are linked by a spacer (calichearubicin A lacks this spacer). The aglycone serves as an anthracycline-like intercalator, while the carbohydrate portion binds to the minor groove of DNA. Thus calichearubicin B exhibits properties of both parent compounds.
are linked by a spacer (calichearubicin A lacks this spacer). The aglycone serves as an anthracycline-like intercalator, while the carbohydrate portion binds to the minor groove of DNA. Thus calichearubicin B exhibits properties of both parent compounds.
Total Synthesis of (–)-Epothilone A†
- Pages: 2801-2803
- First Published: December 1996
An audacious macroaldolization is the key step in the first total synthesis of the cytotoxic epothilone A, which starts with the units 1, 2, and 3. This condensation closes the ring to form the macrolide, whose structure was determined and published (also in Angewandte Chemie) as recently as July 1996.
A Molecularly Defined, Grafted Olefin Metathesis Catalyst from Tris(neopentyl)-nitridomolybdenum(VI)†
- Pages: 2803-2805
- First Published: December 1996
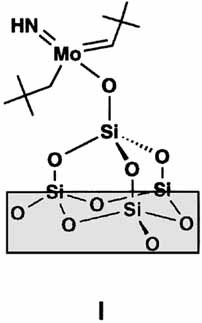
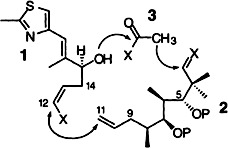
1,2-Addition of the surface silanol groups on SiO2 to the nitridomolybdenum fragment of the title compound and subsequent α-elimination of neopentane forms the catalytically active surface species I. This is the first case in which an alkylated complex is converted into a heterogeneous catalyst in a well-defined way. The immobilized compound is significantly more active in ring-opening metathesis polymerization than the molecular precursor.
Chiral Heterocylic Carbenes in Asymmetric Homogeneous Catalysis†
- Pages: 2805-2807
- First Published: December 1996
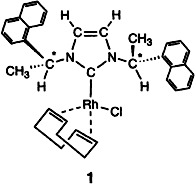
The ee values of the hydrosilylation of acetophenone with the rhodium catalyst 1 are only small; nevertheless, the robustness of the catalyst is impressive: The chiral carbene ligand, which is readily available and can be easily varied, remains bound to the metal in solution to over 100°C. Thus, no excess of ligand is necessary in the catalytic reactions.
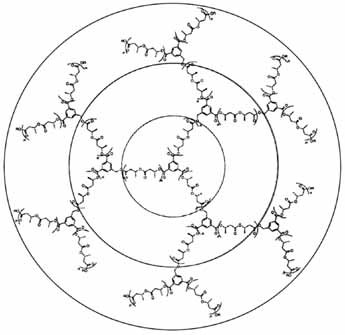
Supramolecular Host–Guest Compounds with Tripod–Metal Templates as Building Blocks at the Corners†‡
- Pages: 2808-2809
- First Published: December 1996
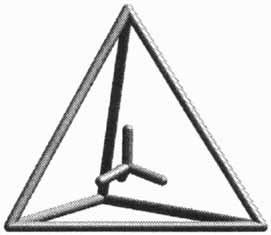
In a one-pot reaction from 15 components, a tetra-hedral host-guest compound forms by self-assembly. Its tripod–iron(II) groups are the corners of a tetrahedron (depicted on the right). The edges of the tetrahedron are bridged by 1,2-dinitrile ligands, and a BF ion is encapsulated in the tetrahedral cavity.
ion is encapsulated in the tetrahedral cavity.
Smaller Substituents on Nitrogen Facilitate the Osmium-Catalyzed Asymmetric Aminohydroxylation†‡
- Pages: 2810-2813
- First Published: December 1996
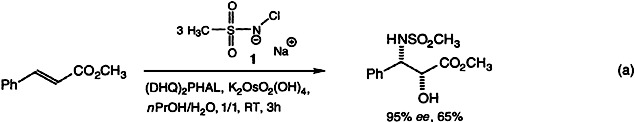
All of the selectivity criteria for catalytic asymmetric aminohydroxylation with sulfonamides “Eq. (a)” are improved when the original nitrogen source, Chloramine-T, is replaced by its methyl analogue, Chloramine-M (1). This newly introduced reagent exhibits substantially higher ligand dependence, and for most substrates the desirable phenomenon of ligand-accelerated catalysis was observed. DHQ-H = dihydroquinine, PHAL = 1,3-phthalazinediyl.
N-Halocarbamate Salts Lead to More Efficient Catalytic Asymmetric Aminohydroxylation†‡
- Pages: 2813-2817
- First Published: December 1996
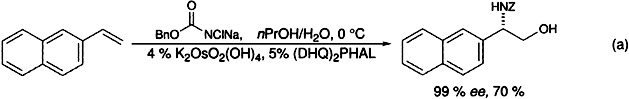
Better enantioselectivities and convenient deprotection make the sodium salts of N-chlorocarbamates the oxidant/nitrogen source of choice for the osmium/cinchona alkaloid catalyzed asymmetric aminohydroxylation (AA) of olefins [e.g. Eq. (a)]. This methodology simplifies the synthesis of a vast array of compounds such as unnatural amino acids and other pharmacologically important compounds. DHQH = dihydroquinine, PHAL = 1,3-phthalazinediyl, Z = benzyloxycarbonyl.
Theory Rules Out a [2 + 2] Addition of Osmium Tetroxide to Olefins as Initial Step of the Dihydroxylation Reaction†
- Pages: 2817-2820
- First Published: December 1996
![Theory Rules Out a [2 + 2] Addition of Osmium Tetroxide to Olefins as Initial Step of the Dihydroxylation Reaction](/cms/asset/b9a25962-c839-450e-9cf7-3f0bb88d28cd/must001.jpg)
A difference of at least 35 kcalmol−1 between the activation barriers results from ab initio calculations for the competing [2 + 2] and [3 + 2] additions of OsO4 to ethylene, clearly favoring the latter pathway (the two transition state structures are pictured on the right). The relative barrier heights for the proposed reaction paths hardly change in the presence of NH3, used to model the base-catalyzed reaction. These theoretical findings on asymmetric dihydroxylation contradict the conclusions drawn from kinetic studies by Sharpless et al., but support the recent kinetic investigations by Corey et al.
5-exo or 6-endo? Exploring Transition State Structures in Cyclizations of 4-Penten-1-oxyl Radicals†‡
- Pages: 2820-2823
- First Published: December 1996
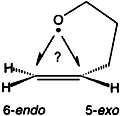
An exo:endo ratio of 98:2! Thermochemistry would have predicted the opposite, but in the rearrangements of 5-hexenyl radicals the exo pathway is preferred due to kinetic reaction control. Selected ab initio methods are able to properly describe cyclizations of the 4-penten-1-oxyl radical (see sketch).
Recognition of the Helical Sense of Polypeptides by a Chiral Metalloporphyrin Receptor†
- Pages: 2823-2825
- First Published: December 1996
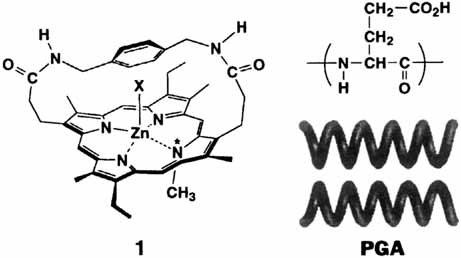
The secondary structure is of primary importance! The D and L forms of α-helical poly(glutamic acid) (PGA) are complexed selectively by the (R) and (S) isomers, respectively, of the porphyrin-based, chiral receptor 1, which has a xylylenediamide strap. Helical polypeptides can thus be separated by complexation with enantiomerically pure 1.
(Alkylidyne)tungsten Complexes Anchored to a Planar Tetraoxo Surface: Exhaustive Alkylation of a (Calixarene)tungsten Complex†
- Pages: 2825-2827
- First Published: December 1996
The First Stable Copper(III) Complex Containing Aliphatic Thiolates as Ligands: Structural and Spectroscopic Evidence for CuII and CuIII Ions in Complexes with Square-Planar CuN2S2 Coordination Environments†‡
- Pages: 2827-2830
- First Published: December 1996
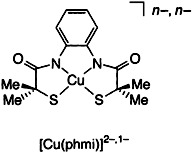
Although it was once deemed a nearly impossible task (since CuII centers are generally readily reduced by thiolates), authentic CuII and CuIII complexes with aliphatic thiolates in the metal coordination sphere have been synthesized. The unusual pair of squareplanar complexes (NEt4)2[Cu(phmi)] · H2O and (PPh4)[Cu(phmi)] (the anion is depicted on the right) displays the lowest known redox potential ever recorded for a CuIII/CuII redox couple and has been completely characterized. H4phmi = N, N′-1,2-phenylenebis(2-mercapto-2-methylpropionamide).
Formal Total Synthesis of (—)-Morphine by Cuprate Conjugate Addition†
- Pages: 2830-2832
- First Published: December 1996
Pyrazolate “Golden” Rings: Trinuclear Complexes That Form Columnar Mesophases at Room Temperature†
- Pages: 2832-2835
- First Published: December 1996
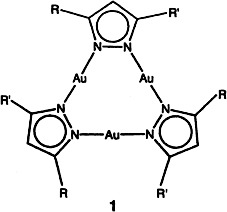
Trinuclear gold(I) pyrazolate complexes of type 1 show liquid crystalline phases at room temperature. The molecules are arranged in a hexagonal columnar structure. The mesogenic behavior of these compounds is strongly related to the molecular symmetry, which is determined by the substituents R and R′. R = 3,4-(H21C10O)2C6H3, R′ = R, 3,4,5-(H21C10O)3C6H2.
Substrate-Directed Diastereoselective Hydroformylation of Methallylic Alcohols†
- Pages: 2835-2837
- First Published: December 1996
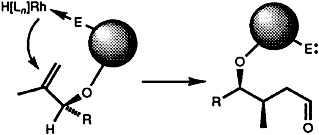
A reversibly coordinating auxiliary is the key to the first highly diastereo-selective hydroformylation of acyclic methallylic alcohols (syn:anti up to 96:4; in the representation on the right the auxiliary is shown as a sphere). The reaction can be used for the efficient construction of stereotriades, the central structural units of polyketide natural products. R is, for example, Ph, CO2Me, Me, CH2Ph.
The Ca2+ Ion as a Cofactor for a Novel RNA-Cleaving Deoxyribozyme†
- Pages: 2837-2841
- First Published: December 1996
In vitro selection experiments yield unexpected results. An RNA-cleaving DNA enzyme that was selected in the presence of Mg2+ ions is much more efficient with Ca2+ as the cofactor. The result is even more surprising because of the lower hydrolytic efficiency of Ca2+ itself.
A Planar [15]Metallacrown-5 That Selectively Binds the Uranyl Cation†
- Pages: 2841-2843
- First Published: December 1996
First Phenylene Polymers Linked by Sulfonium Groups
- Pages: 2843-2845
- First Published: December 1996
Vinyl Glycosides in Oligosaccharide Synthesis: A Strategy for the Preparation of Trisaccharide Libraries Based on Latent-Active Glycosylation†
- Pages: 2845-2847
- First Published: December 1996

Many different trisaccharides (theoretically 256) can in principle be constructed from only four protected building blocks by following the strategy described for the synthesis of trisaccharide libraries. The method relies on latent-active glycosylation. The formula below gives an idea of the compounds in these libraries.
Unusual Regioselectivity in the Reaction of Allyl- or Allenylpropargyltitanium Compounds with Carbonyl Compounds: An Efficient Synthesis of Alkylidenecyclopropane Derivatives†
- Pages: 2848-2849
- First Published: December 1996
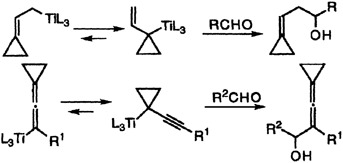
Steric strain shifts the equilibrium between the two isomeric allyltitanium compounds and isomeric allenyl- and propargyltitanium compounds containing cyclopropane units. This leads to unusual regioselectivity in their additions to electrophilic carbonyl compounds (shown on the right) and thus provides easy access to alkylidenecyclopropane derivatives.
Diastereoselective Addition of n-Butyllithium to 2-Phenylpropanal: A Reassessment of the Solvent and Temperature Effects†
- Pages: 2849-2852
- First Published: December 1996
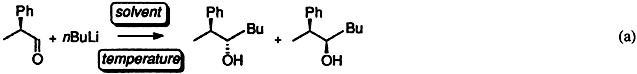
The length of the linear hydrocarbon chain in the solvent is a key factor in the facial diastereoselectivity of the nucleophilic addition of n-butyllithium to 2-phenyl-propanal [Eq. (a)]. The reaction in these solvents shows inversion temperatures that correlate with the melting point of the solvent.
Synthesis and Structure of the Metalated Zintl Ion[Ge9(μ10-Ge)Ni(PPh3)]2−
- Pages: 2852-2854
- First Published: December 1996
![Synthesis and Structure of the Metalated Zintl Ion[Ge9(μ10-Ge)Ni(PPh3)]2−](/cms/asset/31b98c3d-38d5-4d60-84cb-a7e164c8662d/must001.jpg)
The first metalated germanide ion and only the second example of a cluster of the rare nido-10 (iv+iv) structural type, the anion 1, was isolated from the reaction of [Ni(CO)2(PPh3)2], K4Ge9, and “2.2.2”cryptand in ethylenediamine (en). However, the structure of the Ge10Ni cluster (see picture) is distinctly distorted relative to the ideal nido-10 (iv+iv) geometry.
Te6, [Te8Cl18]2−, and [TeCl3]−: New Tellurium and Chlorotellurato Ligands in the Re6 Solid-State Cluster Compounds Re6Te16Cl18 and Re6Te16Cl6†‡
- Pages: 2854-2856
- First Published: December 1996
![Te6, [Te8Cl18]2−, and [TeCl3]−: New Tellurium and Chlorotellurato Ligands in the Re6 Solid-State Cluster Compounds Re6Te16Cl18 and Re6Te16Cl6](/cms/asset/da880c8b-5049-4b57-9163-7ca01f7e3b1f/mgra001.jpg)
Re6 octahedra inside Te8 cubes is a common feature of both title compounds. The picture on the right shows a section of the structure of Re6Te16Cl18, in which the Re6 octahedron and the binding of the cluster to the [Te8Cl18]2− ligand are apparent. Different binding to ligands results in a three-dimensional structure for Re6Te16Cl18, and a two-dimensional structure for Re6Te16Cl6.
First Experimental Determination of the Ionization Potentials of Berkelium and Californium†
- Pages: 2856-2858
- First Published: December 1996
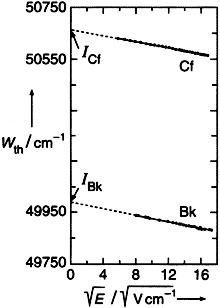
Only 1012 atoms of an element are required for the determination of its first ionization potential by resonance ionization with laser light in the presence of an electric field and subsequent mass spectrometry. The figure on the right shows the ionization thresholds Wth of berkelium and californium and the extrapolation to the external field strength E = 0, which yields the ionization potentials I (49989(2) and 50665(2) cm−1, respectively).
From Pagodanes to Nonpentagonal (Homo)Dodecahedranes—The Undecacyclo-[10.10.0.02,20.03,10.04,19.05,9.06,18.07,15.08,13.019,22.016,21]docosane Cage†‡
- Pages: 2858-2861
- First Published: December 1996
![From Pagodanes to Nonpentagonal (Homo)Dodecahedranes—The Undecacyclo-[10.10.0.02,20.03,10.04,19.05,9.06,18.07,15.08,13.019,22.016,21]docosane Cage](/cms/asset/978c2684-b7d8-4c3f-9082-95e05e6fa27a/must001.jpg)
Twofold, transannular cyclization of the bis(methylene)bisseco intermediates B, obtained from the pagodane diester A, provides access to the parent nonpentagonal bis(homo)dodecahedrane C and pairwise symmetrically functionalized derivatives. Some of these products are important model compounds for quantifying the factors that determine the transannular electronic interactions in cage compounds of this type.
Aggregation of Modified Zinc Chlorins in Nonpolar Solvents-Bacteriochlorophyll c Mimics with Interchanged Hydroxy and Carbonyl Functions†
- Pages: 2861-2863
- First Published: December 1996
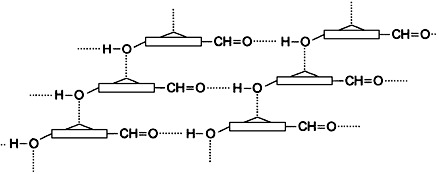
Self-assembly of functionally “inverse” zinc chlorins—31-hydroxy and 131keto groups, which are functionally essential in the bacteriochlorophylls c, d, and e, have been interchanged—is possible in nonpolar solvents (see schematic representation below). This result sets structural conditions for the formation of large aggregates similar to those formed by bacteriochlorophylls in vitro and in light-harvesting antenna systems of photosynthetic bacteria.
New Imido-Bridged Transition Metal Clusters: [(C5H5)4Ti4(NSnMe3)4], [Co11(PPh3)3(NPh)12], [Ni11Br6(NtBu)8], and [Li(thf)4]4[Cu24(NPh)14]†‡
- Pages: 2863-2866
- First Published: December 1996
Monitoring the Reaction Progress in Combinatorial Chemistry: 1H MAS NMR Investigations on Single Macro Beads in the Suspended State†
- Pages: 2867-2869
- First Published: December 1996
Laborious cleavage and isolation steps are avoided! The method of 1H magic angle spinning (MAS) NMR spectroscopy can be used to monitor the course of the synthesis on a single resin particle in a suspended sample in the nanoliter detection range. This extremely useful technique for combinatorial chemistry was tested on a hydantoin reaction sequence.
A Layer Silicate: Synthesis and Structure of the Zeolite Precursor RUB-15—[N(CH3)4]8[Si24O52(OH)4]·20 H2O†
- Pages: 2869-2872
- First Published: December 1996
![A Layer Silicate: Synthesis and Structure of the Zeolite Precursor RUB-15—[N(CH3)4]8[Si24O52(OH)4]·20 H2O](/cms/asset/9ff6d8ef-55e9-49fb-a691-981a03735adf/mgra001.jpg)
The missing link in the series clathrate hydrates, group silicate hydrates—layer silicate hydrates—porous framework silicas is provided by RUB-15, a new layer silicate hydrate with a three-dimensional bonding network composed of layerlike silicate ions and water molecules linked by hydrogen bonds. A section of the structure of is shown on the right. RUB-15 further illustrates the close relationship between crystalline compounds of water, silica, and mixtures thereof.
Synthesis and Characterization of the Stable Dicarbonyl(cyclopentadienyl)iron Radical [(C5R5)Fe(CO)2] (R CHMe2)†‡
- Pages: 2872-2875
- First Published: December 1996
[As2(AlCp*)3]—A Compound with a Polyhedral As2Al3 Framework†
- Pages: 2875-2877
- First Published: December 1996
![[As2(AlCp*)3]—A Compound with a Polyhedral As2Al3 Framework](/cms/asset/b2bf6a52-cacd-40f3-b16e-a285c67adfbe/must001.jpg)
The bonding in heteropolyhedral compounds of the closo-borane type is discussed for “As2(AlCp*)3” clusters, whose molecular structure has been characterized by X-ray crystallography (framework shown on the right). Quantum chemical calculations on several trigonal-bipyramidal E E
E structures revealed the mode of bonding for a variety of ligands (Cp* = C5Me5; EIII: Group 13 element, EV: Group 15 element).
structures revealed the mode of bonding for a variety of ligands (Cp* = C5Me5; EIII: Group 13 element, EV: Group 15 element).
Functionalized Organoimidorhenium(V) Complexes as Potential Radiopharmaceuticals: Syntheses of Glycine Derivatives and the Structure Determination of a Rhenium Analogue of Chlorambucil†
- Pages: 2877-2879
- First Published: December 1996
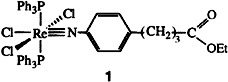
Two important aspects, the synthesis of imido complexes of radioactive metal isotopes and the diversity that can be incorporated with this ligand, distinguish this communication. The multiply bonded organoimide ligand provides a new and powerful means of conjugating suitable radioisotopes of rhenium to biologically relevant molecules in the design of radiopharmaceuticals. Here stable isotope and 188Re-labeled analogues (1) of the anticancer drug chlorambucil were synthesized.
Solvent-Dependent CN Bond Lengths in a Protonated Orthoamide†
- Pages: 2879-2881
- First Published: December 1996





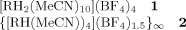
![A Planar [15]Metallacrown-5 That Selectively Binds the Uranyl Cation](/cms/asset/a2147eed-a7c4-464a-9744-75ab6a671d10/must001.jpg)
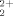 preferentially (see picture), as competition experiments with Ca2+ and Cu2+ showed. This ligand thus provides an alternative to expanded porphyrins for the complexation of actinides and lanthanides by high denticity, planar ligands.
preferentially (see picture), as competition experiments with Ca2+ and Cu2+ showed. This ligand thus provides an alternative to expanded porphyrins for the complexation of actinides and lanthanides by high denticity, planar ligands. 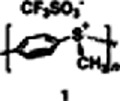

![New Imido-Bridged Transition Metal Clusters: [(C5H5)4Ti4(NSnMe3)4], [Co11(PPh3)3(NPh)12], [Ni11Br6(NtBu)8], and [Li(thf)4]4[Cu24(NPh)14]](/cms/asset/69a419a9-cfff-4df2-8265-23d68929d3ea/must001.jpg)
![Synthesis and Characterization of the Stable Dicarbonyl(cyclopentadienyl)iron Radical [(C5R5)Fe(CO)2] (R CHMe2)](/cms/asset/f7a56ea3-1d6e-42e4-b0c4-9213b50acaa5/must001.jpg)