Vinyl Glycosides in Oligosaccharide Synthesis: A Strategy for the Preparation of Trisaccharide Libraries Based on Latent-Active Glycosylation†
Corresponding Author
Dr. Geert-Jan Boons
School of Chemistry, The University of Birmingham Edgbaston, Birmingham B15 2TT (UK), Fax: Int. code +(121)414-4403, e-mail: [email protected]
School of Chemistry, The University of Birmingham Edgbaston, Birmingham B15 2TT (UK), Fax: Int. code +(121)414-4403, e-mail: [email protected]Search for more papers by this authorDr. Barbra Heskamp
School of Chemistry, The University of Birmingham Edgbaston, Birmingham B15 2TT (UK), Fax: Int. code +(121)414-4403, e-mail: [email protected]
Search for more papers by this authorFloris Hout
School of Chemistry, The University of Birmingham Edgbaston, Birmingham B15 2TT (UK), Fax: Int. code +(121)414-4403, e-mail: [email protected]
Search for more papers by this authorCorresponding Author
Dr. Geert-Jan Boons
School of Chemistry, The University of Birmingham Edgbaston, Birmingham B15 2TT (UK), Fax: Int. code +(121)414-4403, e-mail: [email protected]
School of Chemistry, The University of Birmingham Edgbaston, Birmingham B15 2TT (UK), Fax: Int. code +(121)414-4403, e-mail: [email protected]Search for more papers by this authorDr. Barbra Heskamp
School of Chemistry, The University of Birmingham Edgbaston, Birmingham B15 2TT (UK), Fax: Int. code +(121)414-4403, e-mail: [email protected]
Search for more papers by this authorFloris Hout
School of Chemistry, The University of Birmingham Edgbaston, Birmingham B15 2TT (UK), Fax: Int. code +(121)414-4403, e-mail: [email protected]
Search for more papers by this authorVinyl Glycosides in Oligosaccharide Synthesis, Part 3. Part 2: ref. [7b]. Financial support for this project was provided by the BBSRC (Biotechnical and Biological Sciences Research Council).
Graphical Abstract
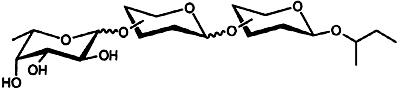
Many different trisaccharides (theoretically 256) can in principle be constructed from only four protected building blocks by following the strategy described for the synthesis of trisaccharide libraries. The method relies on latent-active glycosylation. The formula below gives an idea of the compounds in these libraries.
References
- 1(a) A. Varki, Glycobiology, 1993, 3, 97; (b) R. A. Dwek, Chem. Rev. 1996, 96, 683.
- 2(a)
M. A. Gallop,
R. W. Barrett,
W. J. Dower,
S. P. A. Fodor,
E. M. Gordon,
J. Med. Chem.
1994,
37, 1233;
(b)
M. A. Gallop,
R. W. Barrett,
W. J. Dower,
S. P. A. Fodor,
E. M. Gordon,
Med. Chem.
1994,
37, 1385;
10.1021/jm00035a001 Google Scholar(c) N. K. Terrett, M. Gardner, D. Gordon, R. J. Kobylecki, J. Steele, Tetrahedron 1995, 51, 8135.
- 3 In this paper we use the term combinatorial carbohydrate chemistry to describe the combinatorial synthesis of oligosaccharides. Other authors have employed the same term for the preparation of modified monosaccharide libaries: M. Goebel, I. Ugi, Tetrahedron Lett. 1995, 36, 6043.
- 4
O. Kanie,
F. Barresi,
Y. Ding,
J. Labbe,
A. Otter,
L. S. Forsberg,
B. Ernst,
O. Hindsgaul,
Angew. Chem.
1995,
107, 2912;
10.1002/ange.19951072329 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 2720.
- 5 M. J. Sofia, Drugs Discovery Today 1996, 1, 27.
- 6(a)
H. Paulsen,
Angew. Chem.
1982,
94, 184;
Angew. Chem. Int. Ed. Engl.
1982,
21, 155;
(b)
R. R. Schmidt,
Angew. Chem. Int. Ed. Engl.
1986,
98, 213 and
1986,
25, 212;
(c)
B. Fraser-Reid,
U. E. Udodong,
Z. Wu,
H. Ottoson,
J. R. Merritt,
S. Rao,
C. Roberts,
R. Madsen,
Synlett,
1992,
12, 927;
10.1055/s-1992-21543 Google Scholar(d) P. Fugedi, P. J. Garegg, H. Löhn, T. Norberg, Glycoconjugate J. 1987, 4, 97; (e) K. Toshima, K. Tatsuta, Chem. Rev. 1993, 93 1503; (f) G. J. Boons, Tetrahedron, 1996, 52, 1095.
- 7(a) G. J. Boons, S. Isles, Tetrahedron Lett. 1994, 35, 3593; (b) G. J. Boons, S. Isles, J. Org. Chem 1996, 61, 4262–4271.
- 8 When a glycosylation is performed in acetonitrile at low temperature mainly the β-anomer is obtained, (a) J. Pougny, P. Sinay, Tetrahedron Lett. 1976, 4073; (b) R. U. Lemieux, R. M. Ratcliffe, Can. J. Chem., 1979, 57, 1244; (c) R. R. Schmidt, J. Michel, J. Carbohydr. Chem. 1985, 1, 441; (d) A. J. Rattcliffe, B. Fraser-Reid, J. Chem. Soc. Perkin Trans 1. 1990, 747; (e) Y. D. Vankar, P. S. Vankar, M. Behrendt, R. R. Schmidt, Tetrahedron Lett. 1991, 47, 9985. This anomeric selectivity is lost when such a reaction is performed at ambient temperature. In individual glycosylations, the disaccharides 12-15 were fucosylated, and as expected, each glycosidic linkage was formed as a mixture of anomers. Apart from the data presented here, we have performed many other glycosylations under these conditions and in each case mixtures of anomers have been obtained (unpublished data).
- 9 J. W. Metzger, K. H. Wiesmüller, V. Gnau, J. Brunjes, G. Jung, Angew. Chem. 1993, 105, 901; Angew. Chem. Int. Ed. Engl. 1993, 32, 894.
- 10 M. R. Hardy, R. R. Towdend, Y. C. Lee, Anal. Biochem. 1988, 170, 54.
- 11 The prepared library has a high fucose content and consists of trisaccharides with two different masses (see ref. [13]).
- 12 G. J. Boons, S. Isles, A. Burton, Chem Commun 1996, 141.
- 13 Peaks were observed at m/z 1221 and 1327 ([M + Na]+), corresponding to trisaccharides with two and one fucose units, respectively. Peaks arising from the related disaccharides at m/z 872 and 978 were not detected.
- 14 In the FAB mass spectrum the expected peaks were present at m/z 551 and 567 ([M + Na]+).
- 15 See Dionex technical note no. 20.
- 16 Expected product ratios Fuc/Glc/Gal: 1.0/0.8/0.15; found: 1.0/0.9/0.2. The disaccharide library was prepared from equimolar amounts of disaccharides.