Supramolecular Host–Guest Compounds with Tripod–Metal Templates as Building Blocks at the Corners†‡
Susanne Mann
Anorganisch-chemisches Institut der Universität, Im Neuenheimer Feld 270, D-69120 Heidelberg (Germany), Fax: Int. code +(6221)545707
Search for more papers by this authorProf. Dr. Gottfried Huttner
Anorganisch-chemisches Institut der Universität, Im Neuenheimer Feld 270, D-69120 Heidelberg (Germany), Fax: Int. code +(6221)545707
Search for more papers by this authorCorresponding Author
Dr. Laszlo Zsolnai
Anorganisch-chemisches Institut der Universität, Im Neuenheimer Feld 270, D-69120 Heidelberg (Germany), Fax: Int. code +(6221)545707
Anorganisch-chemisches Institut der Universität, Im Neuenheimer Feld 270, D-69120 Heidelberg (Germany), Fax: Int. code +(6221)545707Search for more papers by this authorKatja Heinze
Anorganisch-chemisches Institut der Universität, Im Neuenheimer Feld 270, D-69120 Heidelberg (Germany), Fax: Int. code +(6221)545707
Search for more papers by this authorSusanne Mann
Anorganisch-chemisches Institut der Universität, Im Neuenheimer Feld 270, D-69120 Heidelberg (Germany), Fax: Int. code +(6221)545707
Search for more papers by this authorProf. Dr. Gottfried Huttner
Anorganisch-chemisches Institut der Universität, Im Neuenheimer Feld 270, D-69120 Heidelberg (Germany), Fax: Int. code +(6221)545707
Search for more papers by this authorCorresponding Author
Dr. Laszlo Zsolnai
Anorganisch-chemisches Institut der Universität, Im Neuenheimer Feld 270, D-69120 Heidelberg (Germany), Fax: Int. code +(6221)545707
Anorganisch-chemisches Institut der Universität, Im Neuenheimer Feld 270, D-69120 Heidelberg (Germany), Fax: Int. code +(6221)545707Search for more papers by this authorKatja Heinze
Anorganisch-chemisches Institut der Universität, Im Neuenheimer Feld 270, D-69120 Heidelberg (Germany), Fax: Int. code +(6221)545707
Search for more papers by this authorWe thank Prof. D. Sellmann and Dr. M. Moll for the 19F NMR spectroscopic measurements.
Dedicated to Professor Walter Siebert on the occasion of his 60th birthday
Graphical Abstract
In a one-pot reaction from 15 components, a tetra-hedral host-guest compound forms by self-assembly. Its tripod–iron(II) groups are the corners of a tetrahedron (depicted on the right). The edges of the tetrahedron are bridged by 1,2-dinitrile ligands, and a BF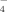 ion is encapsulated in the tetrahedral cavity.
ion is encapsulated in the tetrahedral cavity.
References
- 1
H. A. Mayer,
Chem. Rev.
1994,
94, 1239;
M. Di Varia,
M. Sacconi,
Angew. Chem.
1982,
94, 338;
10.1002/ange.19820940503 Google ScholarAngew. Chem. Int. Ed. Engl. 1982, 21, 330; M. Di Vaira, F. Mani, S. Moneti, M. Peruzzini, L. Sacconi, P. Stoppioni, Inorg. Chem. 1985, 24, 2230; C. Bianchini, C. Mealli, A. Meli, M. Sabat, P. Zanello, J. Am. Chem. Soc. 1987, 109, 185.
- 2 M. Di Vaira, S. Midollini, L. Sacconi, J. Am. Chem. Soc. 1979, 101, 1757; C. Mealli, S. Midollini, L. Sacconi, Inorg. Chem. 1975, 14, 2513; A. Barth, G. Huttner, M. Fritz, L. Zsolnai, Angew. Chem. 1990, 102, 956; Angew. Chem. Int. Ed. Engl. 1990, 29, 929; S. Vogel, A. Barth, G. Huttner, L. Zsolnai, R. Kremer, Angew. Chem. Int. Ed. Engl. 1991, 103, 325 and. 1991, 30, 303.
- 3 C. A. Ghilardi, F. Laschi, S. Midollini, A. Orlandini, G. Scapacci, P. Zanello, J. Chem. Soc. Dalton Trans. 1995, 4, 531.
- 4 P. Dapporto, S. Midollini, L. Sacconi, Inorg. Chem 1975, 14, 1643.
- 5 V. Körner, A. Asam, G. Huttner, L. Zsolnai, M. Büchner, Z. Naturforsch. B 1994, 49, 1183.
- 6 A. Asam, B. Janssen, G. Huttner, L. Zsolnai, O. Walter, Z. Naturforsch. B 1994, 48, 1707.
- 7 A. Muth, O. Walter, G. Huttner, A. Asam, L. Zsolnai, C. Emmerich, J. Organomet. Chem. 1994, 468, 149.
- 8
R. W. Saalfrank,
R. Burak,
A. Breit,
D. Stalke,
R. Herbst-Irmer,
J. Daub,
M. Porsch,
E. Bill,
M. Müther,
A. X. Trautwein,
Angew. Chem.
1994,
106, 1697;
Angew. Chem. Int. Ed. Engl.
1994,
33, 1621;
R. W. Saalfrank,
Angew. Chem. Int. Ed. Engl.
1995,
107, 1085 and
1995,
34, 993;
10.1002/ange.19951070911 Google ScholarB. Dietrich, Pure Appl. Chem. 1993, 65, 1457; M. Fujita, S. Nagao, K. Ogura, J. Am. Chem. Soc. 1995, 117, 1649; P. Baxter, J. M. Lehn, A. DeCian, J. Fischer, Angew. Chem. 1993, 105, 92; Angew. Chem. Int. Ed. Engl. 1993, 32, 69; K. Worm, F. P. Schmidtchen, A. Schier, A. Schäfer, M. Hesse, Angew. Chem. Int. Ed. Engl. 1994, 106, 360, and 1994, 33, 327; F. P. Schmidtchen, Nachr. Chem. Tech. Lab. 1988, 36, 8; A. Müller, H. Reuter, S. Dillinger, Angew. Chem. 1995, 107, 2505; Angew. Chem. Int. Ed. Engl. 1995, 34, 2328; X. Yang, C. B. Knobler, M. F. Hawthrone, Angew. Chem. Int. Ed. Engl. 1991, 103, 1519 and 1991, 30, 1507; M. Newcomb, J. H. Horner, M. T. Blanda, P. J. Squattrito, J. Am. Chem. Soc. 1989, 111, 6294; T. Beissel, R. E. Powers, K. N. Raymond, Angew. Chem. 1996, 108, 1166;10.1002/ange.19961081008 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 1084.
- 9 1a·[BF4]7: 0.20 × 0.30 × 0.30 mm; orthorhombic; space group Aba2; Z = 4; a = 2766(1), b = 3007(1), c = 2908(2) pm; V = 24190 × 106 pm3; ρcalcd = 1.127 g cm−3; measurement range 2.7 ≤ 2θ ≤ 54.0°; 13481 measured reflections, of which 13481 were independent; 5583 observed (I ≥ 2σ(I)); R = 0.138, Rw = 0.434, residual electron density 1.23 × 10−6epm−3. 1b·(BF4)7: 0.30 × 0.30 × 0.30 mm; orthorhombic; space group Aba2; Z = 4; a = 2743.2(6), b = 2975(2), c = 2866.9(8) pm; V = 23396 × 106 pm3; ρcalcd = 1.215 g cm−3; measurement range 4.1 ≤ 2θ ≤ 47.0°; 8751 measured reflections, of which 8751 independent; 6932 observed (I ≥ 2σ(I)); R = 0.094; Rw = 0.278; residual electron density 1.21 × 10−6epm−3. For 1a and 1b: Siemens(Nicolet Syntex) R3m/V Diffractometer, MoKα graphite monochromator, Lorentz and polarization factors; experimental absorption correction; ψ scan; direct methods; SHELXL93 [1], SHELXS-86 [2] (G M. Sheldrick, University Cambridge, 1976); least squares method. Further details of the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, D-76344 Eggenstein-Leopoldshafen (Germany), on quoting the depository numbers CSD-405687 (1a) and CSD-405686 (1b).
- 10 Bond lengths [Å] and angles [°] 1b (cf. Fig. 1): FeN 1.91 – 1.97, FeP 2.25–2.29, Fe1–F1 (Fe1A–F1A) 4.27, Fe2–F2 (Fe2A—- F2A) 4.66, B1–F1 (B1–F1A) 1.39, B1–F2 (B1–F2A) 1.40; N-Fe-N 81.7–85.2, P-Fe-P 88.4–91.0.
- 11 An idealized orthorhombic face-centered unit cell is formed by the translation along thez axis by about 1/4 c.
- 12
H. Funk,
F. Binder,
Z. Anorg. Chem.
1926,
166, 327.
10.1002/zaac.19261550136 Google Scholar





