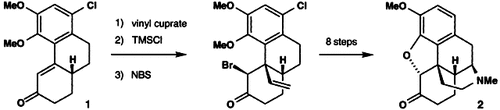
Formal Total Synthesis of (—)-Morphine by Cuprate Conjugate Addition†
Corresponding Author
Prof. Dr. Johann Mulzer
Institut für Organische Chemie der Universität, Währingerstrasse 38, A-1090 Wien (Austria), Fax: Int. code +(1)313672280, e-mail: [email protected]
Institut für Organische Chemie der Universität, Währingerstrasse 38, A-1090 Wien (Austria), Fax: Int. code +(1)313672280, e-mail: [email protected]Search for more papers by this authorDr. Gerd Dürner
Institut für Organische Chemie der Universität, Marie-Curie-Strasse 11, D-60439 Frankfurt am Main (Germany)
Search for more papers by this authorDipl.-Chem. Dirk Trauner
Institut für Organische Chemie der Universität, Währingerstrasse 38, A-1090 Wien (Austria), Fax: Int. code +(1)313672280, e-mail: [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Johann Mulzer
Institut für Organische Chemie der Universität, Währingerstrasse 38, A-1090 Wien (Austria), Fax: Int. code +(1)313672280, e-mail: [email protected]
Institut für Organische Chemie der Universität, Währingerstrasse 38, A-1090 Wien (Austria), Fax: Int. code +(1)313672280, e-mail: [email protected]Search for more papers by this authorDr. Gerd Dürner
Institut für Organische Chemie der Universität, Marie-Curie-Strasse 11, D-60439 Frankfurt am Main (Germany)
Search for more papers by this authorDipl.-Chem. Dirk Trauner
Institut für Organische Chemie der Universität, Währingerstrasse 38, A-1090 Wien (Austria), Fax: Int. code +(1)313672280, e-mail: [email protected]
Search for more papers by this authorDedicated to Professor Wilhelm Fleischhacker on the occasion of his 65th birthday
Graphical Abstract
References
- 1
For excellent reviews of the total synthesis of morphine alkaloids see (a)
T. Hudlicky,
G. Butora,
S. P. Fearnley,
A. G. Gum,
M. R. Stabile in
Studies in Natural Products Chemistry,
Vol. 18
(Ed.: Atta-ur-Rahman),
Elsevier, Amsterdam,
1996, p. 43;
(b)
M. Maier in
Organic Synthesis Highlights II
(Ed.: H. Waldmann),
VCH, Weinheim,
1995, p. 357;
10.1002/9783527619948.ch38 Google Scholar(c) G. Szántay, G. Dörnyei in The Alkaloids, Vol. 45 (Eds.: G. A. Cordell, A. Brossi), Academic Press, New York, 1994, p. 127.
- 2(a) C. Y. Hong, N. Kado, L. E. Overman, J. Am. Chem. Soc. 1993, 115, 11028; (b) K. A. Parker, D. Focas, J. Am. Chem. Soc. 1992, 114, 9688; (c) M. A. Tius, M. A. Kerr, J. Am. Chem. Soc. 1992, 114, 5959; (d) J. E. Toth, P. R. Hamann, P. L. Fuchs, J. Org. Chem 1988, 53, 4694; (e) P. J. Parsons, C. S. Penkett, A. Shell, Chem. Rev. 1996, 96, 195.
- 3(a) Organocopper Reagents, A Practical Approach (Ed.: R. K. Taylor), Oxford University Press, 1994; (b) B. H. Lipshutz in Organometallics in Synthesis, Chap. 4 (Ed.: M. Schlosser), Wiley, Chichester, 1994; (c) B. H. Lipshutz, S. Sengupta, Org. React. 1992, 41, 135; (d) P. Perlmutter, Conjugate Addition Reactions in Organic Synthesis, Pergamon, Oxford, 1992.
- 4 Alternatively, the Eschenmoser–Claisen and [2,3]-Wittig rearrangements were successfully applied. The two strategies could be pursued in parallel, since the conjugate addition is performed with an enone, which on reduction delivers the allylic alcohol necessary for a sigmatropic rearrangement.
- 5 R. Ghosh, R. Robinson, J. Chem. Soc. 1944, 506.
- 6 Flash chromatography; cellulose triacetate from the company Merck (No. 16362); methanol as eluent; 0.5 g 5 per 200 g cellulose triacetate.
- 7 E. Nakamura in ref. [3a]; (b) S. Matsuzawa, Y. Horiguchi, E. Nakamura, I. Kuwajima, Tetrahedron 1989, 45, 349; (c) E. J. Corey, N. W. Boaz, Tetrahedron Lett. 1985, 26, 6019.
- 8 J. Mulzer, J. W. Bats, D. Trauner, Synlett, submitted.
- 9(a) K. E. Harding, B. A. Clement, L. Moreno, J. Peter-Katalinic, J. Org. Chem. 1981, 46, 940; (b) P. M. Wege, R. D. Clark, C. H. Heathcock, J. Org. Chem. 1976, 41, 3144.
- 10(a) M. Ihara, A. Katsumata, M. Egashira, S. Suzuki, Y. Tokunaga, K. Fukumoto, J. Org. Chem. 1995, 60, 5560; (b) M. J. Chapdelaine, M. Hulce, Org. React. 1990, 38, 225.
- 11 T. K. Kawabata, P. A. Grieco, H.-L. Sham, H. Kim, J. Y. Jaw, S. Tu, J. Org. Chem. 1987, 52, 3347.
- 12 T. H. Chan, M. A. Brook, T. Chaly, Synthesis 1983, 203.
- 13 T. Tsunoda, Y. Yamamiya, S. Itǒ, Tetrahedron Lett. 1993, 34, 1639.
- 14 T. Kametani, Y. Suzuki, T. Honda, J. Chem. Soc. Perkin Trans. 1986, 1372.
- 15(a) D. D. Weller, H. Rapoport, J. Med. Chem. 1976, 19, 1173; (b) I. Ijima, J. Minamikawa, A. E. Jacobson, A. Brossi, K. C. Rice, J. Org. Chem. 1978, 43, 1463; (c) K. C. Rice, J. Org. Chem. 1980, 45, 3135.
- 16 J. Mulzer in Houben-Weyl, Methoden der Organischen Chemie, Vol. E21a, Chap. A.2.1 (Eds.: G. Helmchen, R. W. Hoffmann, J. Mulzer, E. Schaumann), Thieme, Stuttgart 1995, p. 91.
- 17 Cf. ref.[2a]: 14 steps (6.5%); ref. [2b]: 13 steps (9.5%); ref. [12c]: 9 steps (29%).