Journal list menu
Export Citations
Download PDFs
Cover Picture (Angew. Chem. Int. Ed. Engl. 16/1996)
- First Published: September 6, 1996
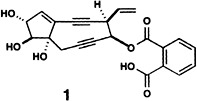
The cover picture shows the crystal structure of a unique supramolecular architecture consisting of five tris(bispyridine) ligands (shown in different colors) wrapped around five FeII ions (purple) in a double-helical fashion and closed to form a torus around a central chloride ion (green). This circular helicate is an analogue of circular DNA. Its formation also points to the possibility of designing self-assembling systems that generate receptors for specific substrates from a collection of suitable ligands and metal ions. More about the circular helicate and the concept of “virtual combinatorial libraries” is reported by J.-M. Lehn et al. on p. 1838 ff.
Graphical Abstract (Angew. Chem. Int. Ed. Engl. 16/1996)
- Pages: 1747-1753
- First Published: September 6, 1996
Reviews
My Life with O3, NOx, and Other YZOx Compounds (Nobel Lecture)†‡
- Pages: 1758-1777
- First Published: September 6, 1996
The ozone hole, a nuclear winter, and the greenhouse effect are some of the themes that concerned Paul Crutzen in his work, for which he was awarded the Nobel Prize in 1995. Here he reports on his path to his path to his results and his plans for the future.
Polar Ozone Depletion (Nobel Lecture)†‡
- Pages: 1778-1785
- First Published: September 6, 1996
Cause and result can be geographically widely separated. This fact is corroborated by the finding that the annually recurring ozone hole over Antarctica, which has been steadily increasing in size since 1985, is predominantly due to anthropogenic emissions from the Northern Hemisphere. How the realization dawned that it is primarily the chlorine atoms released by photochemical reactions of CFCs in the upper stratosphere that destroy the ozone shield is described by M. J. Molina in his Nobel Lecture.
Stratospheric Ozone Depletion by Chlorofluorocarbons (Nobel Lecture)†
- Pages: 1786-1798
- First Published: September 6, 1996
Hope for the atmosphere is the cautiously worded resumé of F. S. Rowland based on his knowledge of the developments in recent years about the influence of the CFCs on the ozone budget. Thanks to the restrictions that came into effect in the 1990s, the concentrations of some CFCs in the atmosphere have already reached their maximum value; for others this point will be reached in the near future. Nevertheless, large ozone losses are expected in the Antarctic spring until the middle of the 21st century.
Communications
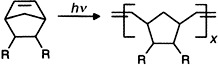
Generation of Cyclocarbons with 4n Carbon Atoms (C12, C16, and C20) by [2 + 2] Cycloreversion of Propellane-Annelated Dehydroannulenes†‡
- Pages: 1800-1802
- First Published: September 6, 1996
1,2-Distanna[2]- and 1,2,3-Tristanna[3]-ferrocenophanes†‡
- Pages: 1803-1804
- First Published: September 6, 1996
![1,2-Distanna[2]- and 1,2,3-Tristanna[3]-ferrocenophanes](/cms/asset/db795666-3677-4fed-9cab-aeb56663564c/must001.jpg)
A convenient route to the stanna[n]ferrocenophanes 1 and 2 is presented. The series of homologous hetero[2]ferrocenophanes of the type [Fe(C5H4EMe2)2] (E = C, Si, Sn) is thus supplemented by 1. A comparison of the molecular structures (for E = C, Si, Sn) reveals that as the EE bond length increases, the strain in the [2]ferrocenophane molecule decreases.
Synthesis and Ring-Opening Polymerization of a Tin-Bridged [1]Ferrocenophane†
- Pages: 1805-1807
- First Published: September 6, 1996
![Synthesis and Ring-Opening Polymerization of a Tin-Bridged [1]Ferrocenophane](/cms/asset/b6e36930-6ddd-4cd5-bca4-6bff456d3b36/must001.jpg)
What is the smallest tilt angle necessary for an [n]metallocenophane to undergo ring-opening polymerization (ROP)? In previous work [n]metallocenophanes with tilt angles of up to 13° were found to be resistant to thermally induced ROP. The stanna[1]ferrocenophane 1, the first [n]metallocenophane containing a heavy main group element in the bridge, has a tilt angle of only 14° but still polymerizes to yield novel high molecular weight poly(ferrocenylstannane)s 2.
Azido and 2,2′-Bipyrimidine Ligands as Useful Tools in Designing Two- and Three-Dimensional Manganese(II) Networks†
- Pages: 1807-1810
- First Published: September 6, 1996
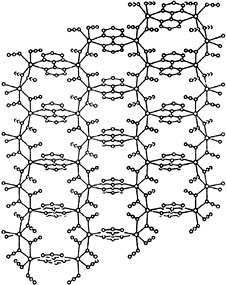
Same composition but different structures characterize the manganese complexes 1 and 2 of the formula [Mn2(bpym)(N3)4], which are obtained by the reaction of manganese(II) nitrate with 2,2′-bipyrimidine (bpym) and sodium azide in aqueous solution. Compound 1 has a honeycomb layered structure (shown on the right) and exhibits overall ferromagnetic interactions; 2 has a three-dimensional polymeric structure and shows overall antiferromagnetic interactions.
Alternating Ferro- and Antiferromagnetic Interactions in Honeycomb-Like Layers of an Azidomanganese(II) Compound†
- Pages: 1810-1812
- First Published: September 6, 1996
Manganese(II) nitrate, sodium azide, and 2,2′-bipyrimidine (bpym) react to give 1 in which the azido groups function as end-on bridging ligands and the bpym as a bis(bidentate) ligand. The structure of 1 can be described as a honeycomb sheet formed by Mn6 hexagons. Magnetic susceptibility data reveals the existence of ferro- and antiferromagnetic interactions that are mediated by the azido ligands and the bpym ligands, respectively.
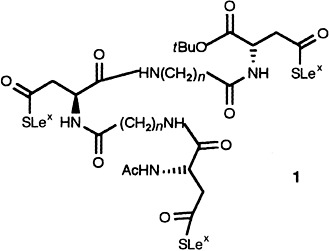
Chemoenzymatic Synthesis of Sialyl Lewisx Glycopeptides†
- Pages: 1812-1815
- First Published: September 6, 1996
Molecular Recognition Analyzed by EPR, ENDOR, and NMR Spectroscopy†
- Pages: 1815-1818
- First Published: September 6, 1996
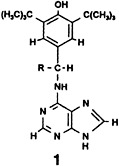
Dynamic systems consisting of heterocyclic substrates like 1 and a model receptor can be resolved by EPR and ENDOR spectroscopy because of the difference in time resolution of these techniques. Intermolecular interactions in these lock-and-key complexation equilibria influence the intramolecular flexibilities. R = CH3.
Bis(bipyridine) Ligands in Manganese Carboxylate Cluster Chemistry: Self-Assembly of a Cluster Complex with Two Butterfly-Like [Mn4(μ3-O)2]8+ Cores†
- Pages: 1818-1820
- First Published: September 6, 1996
![Bis(bipyridine) Ligands in Manganese Carboxylate Cluster Chemistry: Self-Assembly of a Cluster Complex with Two Butterfly-Like [Mn4(μ3-O)2]8+ Cores](/cms/asset/33c765a7-2f36-4f2e-ba97-554db0bafd8c/must001.jpg)
Self-assembly of new tetranuclear (1) and octanuclear (2) manganese carboxylate clusters is controlled by the bis(bipyridine) ligands L1 and L2, respectively, which are shown on the right. The cation 1 consists of two Mn2O complex fragments, each of which contains one MnII and one MnIII center; 2 contains two butterfly-like Mn4O2 cores linked by bis(bipyridine) bridges. Counterion: ClO .
.
Rhodium(I)-Catalyzed Cycloaromatization of Acyclic 3-Ene-1,5-diynes†
- Pages: 1823-1825
- First Published: September 6, 1996
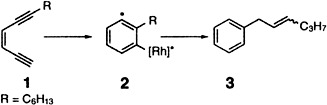
A transition metal catalyst can trigger the cycloaromatization of an enediyne. A coordinatively unsaturated rhodium complex catalyzes the cyclization of the acyclic enediyne 1 to produce 3. The cyclization, which is similar to the Myers reaction, proceeds via a vinylidene–rhodium intermediate and the 1,4-organorhodium diradical 2. Rh = [RhCl(iPr3P)2].
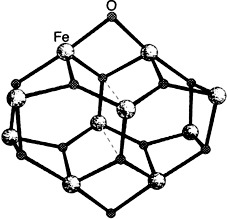
Ferric Wheels and Cages: Decanuclear Iron Complexes with Carboxylato and Pyridonato Ligands†
- Pages: 1825-1828
- First Published: September 6, 1996
Structural Reorganization of the Doubly Protonated [222] Cryptand through Cation–π and Charge–Charge Interactions: Synthesis and Structure of Its [CoCl4]·0.5 C6H5CH3 Salt†
- Pages: 1828-1830
- First Published: September 6, 1996
![Structural Reorganization of the Doubly Protonated [222] Cryptand through Cation–π and Charge–Charge Interactions: Synthesis and Structure of Its [CoCl4]·0.5 C6H5CH3 Salt](/cms/asset/2bbf0455-a5e3-44f1-bd5f-c4b9a0348e7f/must001.jpg)
From an arene-rich liquid clathrate medium the title compound can be isolated in crystalline form. It is the first example of a compound in which an ionophore is stabilized by NH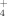 – π interaction. The crystal contains a linear supramolecular complex comprising five components (see picture) in which the cryptate molecules are elongated along their long axes.
– π interaction. The crystal contains a linear supramolecular complex comprising five components (see picture) in which the cryptate molecules are elongated along their long axes.
Enantioselective Synthesis of Copper(I) Bipyridine Based Helicates by Chiral Templating of Secondary Structure: Transmission of Stereochemistry on the Nanometer Scale†
- Pages: 1830-1833
- First Published: September 6, 1996
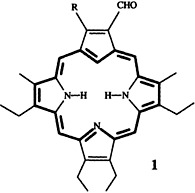
Metalloporphyrins as Ligands: Synthesis and Characterization of [(η6-cymene)-Ru{η5-Ni(OEP)}]2+†
- Pages: 1833-1835
- First Published: September 6, 1996
DNA Cleavage by a Nine-Membered Masked Enediyne, an Analogue of the Kedarcidin and C-1027 Chromophores†
- Pages: 1835-1837
- First Published: September 6, 1996
A phthalate group serves as the trigger for controlling the stability of the enediyne precursor 1. β-Elimination of this trigger generates the corresponding enediyne and the DNA-cleaving biradical. The highly strained nine-membered ring in 1, an analogue of the kedarcidin chromophore, was constructed by a transannular [2,3] Wittig rearrangement. Incubation of supercoiled DNA with 1 (1 mM) leads to double strand cleavage without the addition of a reducing agent, which is required for other DNA cleaving systems.
Self-Assembly of a Circular Double Helicate†‡
- Pages: 1838-1840
- First Published: September 6, 1996
The simple reaction of a tris-bpy ligand strand with FeCl2 leads to the self-assembly of the unique cation 1, which consists of a torus formed by five ligands wrapped around five FeII ions in a double-helical fashion and closed around a central chloride ion. This structure, a fivefold circular helicate, is a helicate analogue of circular DNA.
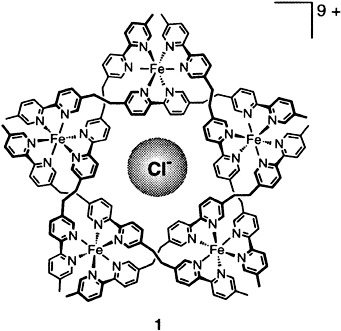
Planar Bischlorophyll Derivatives with a Completely Conjugated π-System: Model Compounds for the Special Pair in Photosynthesis†
- Pages: 1840-1842
- First Published: September 6, 1996
The titanium-mediated coupling of NiII and ZnII pyrophaeophorbides results in formation of planar ethenefused bisphaeophorbides 1, M = Ni, Zn. Absorption spectroscopy indicates that the two chlorin units are highly conjugated, and the X-ray crystal structure shows the two phaeophorbide rings to be almost coplanar.
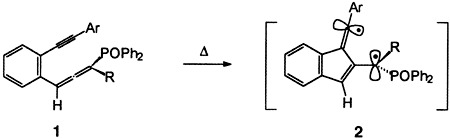
An Unprecedented Biradical Cyclization as an Alternative Pathway to the Myers–Saito Cycloaromatization in the Thermal Reactions of Enyne Allenes†‡
- Pages: 1843-1845
- First Published: September 6, 1996
Photoinduced Ring-Opening Metathesis Polymerization (PROMP) with Photochemically Generated Schrock-Type Catalysts
- Pages: 1845-1847
- First Published: September 6, 1996
A new type of photopolymerization (depicted on the right) relies on well-defined, early transition metal complexes. Schrock-type catalysts are photochemically generated from di- or trialkyl tungsten complexes. The photoactivity and latency of the new catalysts are demonstrated by photo-differential scanning calorimetric and viscosimetric measurements.
Asymmetric Synthesis of Alkyl Aziridine-2-Carboxylates from Chiral 3′-Benzyloxy-aminoimides†
- Pages: 1848-1849
- First Published: September 6, 1996
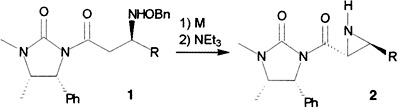
Diastereoselectivities at previously unattained levels. Aziridines 2 can be prepared from precursors 1 via the Ti or Al enolates in > 99% de. Further advantages of this approach are the easy access to 1 by AlMe2Cl-catalyzed 1,4-addition of O-benzylhydroxylamine to α,β-unsaturated chiral imides, and the straightforward recovery of the chiral auxiliary by treatment with lithium benzyloxide. R = Me, Et, nPr; M = TiCl4, AlMe2Cl.
Synthesis of New Hybrid Materials by Intercalation of a Bifunctional Aminophosphane and Its Tungsten Pentacarbonyl Complex in α-Zirconium Phosphate†
- Pages: 1850-1852
- First Published: September 6, 1996
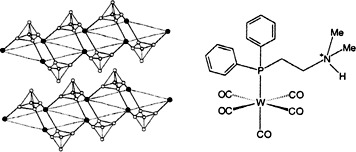
Functionalization of a phosphane ligand facilitates intercalation of the free ligand and its tungsten pentacarbonyl complex (example shown bottom right) in α-zirconium phosphate (section of structure shown bottom left). Syntheses of intercalated products with the stoichiometry [Zr(HPO4)2(Q)x·yH2O] (Q = Ph2PCH2CH2NMe2, x = 0.46, y = 1.1; Q = (OC)5W(Ph2PCH2CH2NMe2), x = 0.51, y = 1.5) are reported.
Nonlinear Temperature Behavior of Product Ratios in Selection Processes†‡
- Pages: 1852-1854
- First Published: September 6, 1996
Displaced intermolecular and intramolecular equilibria, which lead to a nonlinear change in the concentration ratios of intermediates, are possibly the cause for the experimentally long-known nonlinear temperature dependence of logarithmic product ratios in selection processes (isoinversion principle). This interpretation takes into account a change in dominance of differences in the activation enthalpy and entropy for the overall selection process, as well as a change in the rate-determining step with variation of the temperature.
Synthesis of New Dialkylmagnesium Compounds by Living Transfer Ethylene Oligo- and Polymerization with Lanthanocene Catalysts†
- Pages: 1854-1856
- First Published: September 6, 1996
Useful intermediates for the synthesis of functionalized, linear alkyl chains by classical Grignard reactions or reactants for the synthesis of biblock copolymers by further reaction with polar monomers—the dialkylmagnesium compounds described herein are accessible in a living transfer polymerization under very mild conditions with [Cp LnCl2Li(OEt2)2] (Ln = Nd, Sm) as catalysts and simple dialkylmagnesium compounds RMgR′ (R = Et, R′ = Bu or R = R′ = n-hexyl) using ethylene as substrate.
LnCl2Li(OEt2)2] (Ln = Nd, Sm) as catalysts and simple dialkylmagnesium compounds RMgR′ (R = Et, R′ = Bu or R = R′ = n-hexyl) using ethylene as substrate.
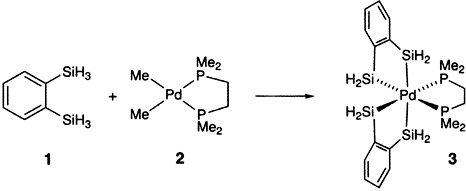
The First Silylpalladium(IV) Complex
- Pages: 1856-1858
- First Published: September 6, 1996
Metallomesogens with Branched, Dendrimeric Amino Ligands†
- Pages: 1858-1861
- First Published: September 6, 1996
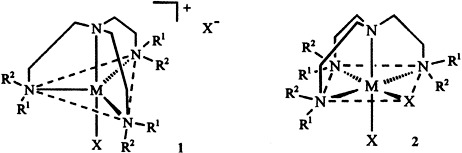
Trigonal-bipyramidal and octahedral coordination is found in transition metal complexes 1 and 2 (M = Co, Ni, Cu, Zn; X = Cl, NO3, NCS (or SCN)) with dendrimeric ethyleneimine ligands of the first (1 = H; R2 = CH2-3,4-C6H3[O(CH2)9CH3]2) and second generation (R1 = R2 = CH2CH2NHCO-3,4-C6H3[O(CH2)9CH3]2). These complexes form a novel class of metallomesogens. With a second generation dendrimer as the ligand or with ZnII as the central atom, a hexagonal-columnar phase is formed. Otherwise, lamellar mesophases are obtained.
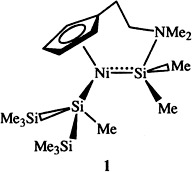
An Intermolecularly Donor-Stabilized Silanediul(silyl)nickel Complex: Combined SiSi Bond Cleavage and Methyl Migration between Silicon Centers†‡
- Pages: 1861-1863
- First Published: September 6, 1996
Synthesis and Asymmetric [3 + 2] Cycloaddition Reactions of Chiral Cyclic Nitrone: A Novel System Providing Maximal Facial Bias for both Nitrone and Dipolarophile†
- Pages: 1863-1864
- First Published: September 6, 1996
![Synthesis and Asymmetric [3 + 2] Cycloaddition Reactions of Chiral Cyclic Nitrone: A Novel System Providing Maximal Facial Bias for both Nitrone and Dipolarophile](/cms/asset/cd6893ff-2e6f-4d6f-9b03-94287d35b9ec/must001.jpg)
Only the convex face of the chiral, cyclic nitrone 1 is accessible to dipolarophiles 2 in [3 + 2] cycloadditions. Since in addition Z2 dictates the orientation of the dipolarophiles by directing the smallest substituent (H) of 2 into the most demanding space, the cycloadducts 3 are obtained with almost perfect stereocontrol.
Book Reviews
Book Review: Hydrocarbon Chemistry. By G. A. Olah and Á. Molnár
- Pages: 1865-1866
- First Published: September 6, 1996
Book Review: Organic Synthesis Highlights II. Edited by H. Waldmann
- Page: 1866
- First Published: September 6, 1996
Book Review: Theoretical and Physical Principles of Organic Reactivity. By A. Pross
- Pages: 1866-1867
- First Published: September 6, 1996
Book Review: Organomagnesium Methods in Organic Synthesis. (Series: Best Synthetic Methods.) By B. J. Wakefield
- Pages: 1867-1868
- First Published: September 6, 1996
Book Review: Multidimensional NMR in Liquids. Basic Principles and Experimental Methods. By F. J. M. van der Ven
- Pages: 1868-1869
- First Published: September 6, 1996
Book Review: Chemistry of Waste Minimization. Edited by J. H. Clark
- Page: 1869
- First Published: September 6, 1996
Book Review: Fifty Years of Free Radicals. (Series: Profiles, Pathways, and Dreams. Series editor: J. I. Seeman) By C. Walling
- Pages: 1869-1871
- First Published: September 6, 1996
Book Review: Symmetry and Structure. Readable Group Theory for Chemists. 2nd edition. By S. F. A. Kettle
- Page: 1871
- First Published: September 6, 1996





![Generation of Cyclocarbons with 4n Carbon Atoms (C12, C16, and C20) by [2 + 2] Cycloreversion of Propellane-Annelated Dehydroannulenes](/cms/asset/0421f2dc-328b-40e0-ad74-cf708385ef5b/must001.jpg)

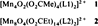
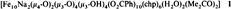
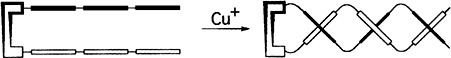
![Metalloporphyrins as Ligands: Synthesis and Characterization of [(η6-cymene)-Ru{η5-Ni(OEP)}]2+](/cms/asset/4ba2e592-1f16-4c6f-82b5-b4dd4f5d5598/must001.jpg)