An Unprecedented Biradical Cyclization as an Alternative Pathway to the Myers–Saito Cycloaromatization in the Thermal Reactions of Enyne Allenes†‡
Corresponding Author
Prof. Dr. Michael Schmittel
Institut für Organische Chemie der Universität, Am Hubland, D-97074 Würzburg (Germany) Fax: Int. code +(931) 888-4606 e-mail: [email protected]
Institut für Organische Chemie der Universität, Am Hubland, D-97074 Würzburg (Germany) Fax: Int. code +(931) 888-4606 e-mail: [email protected]Search for more papers by this authorDipl.-Chem. Marc Strittmatter
Institut für Organische Chemie der Universität, Am Hubland, D-97074 Würzburg (Germany) Fax: Int. code +(931) 888-4606 e-mail: [email protected]
Search for more papers by this authorDipl.-Chem. Susanne Kiau
Institut für Organische Chemie der Universität, Am Hubland, D-97074 Würzburg (Germany) Fax: Int. code +(931) 888-4606 e-mail: [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Michael Schmittel
Institut für Organische Chemie der Universität, Am Hubland, D-97074 Würzburg (Germany) Fax: Int. code +(931) 888-4606 e-mail: [email protected]
Institut für Organische Chemie der Universität, Am Hubland, D-97074 Würzburg (Germany) Fax: Int. code +(931) 888-4606 e-mail: [email protected]Search for more papers by this authorDipl.-Chem. Marc Strittmatter
Institut für Organische Chemie der Universität, Am Hubland, D-97074 Würzburg (Germany) Fax: Int. code +(931) 888-4606 e-mail: [email protected]
Search for more papers by this authorDipl.-Chem. Susanne Kiau
Institut für Organische Chemie der Universität, Am Hubland, D-97074 Würzburg (Germany) Fax: Int. code +(931) 888-4606 e-mail: [email protected]
Search for more papers by this authorThermal and Electron Transfer Induced Reactions of Enediynes and Enyne Allenes, Part 4. Financial support from the Volkswagen-Stiftung and the Fonds der Chemischen Industrie is gratefully acknowledged. We thank Prof. Dr. C. Rüchardt and E. Hickl (Freiburg) for their kind help with the DSC experiments. Part 3: [6].
Dedicated to Professor Horst Prinzbach on the occasion of his 65th birthday
Graphical Abstract
References
- 1(a) R. G. Bergman, R. R. Jones, J. Am. Chem. Soc. 1972, 94, 660–661; (b) A. G. Myers, Tetrahedron Lett. 1987, 28, 4493–4496; (c) A. G. Myers, E. Y. Kuo, N. S. Finney, J. Am. Chem. Soc. 1989, 111, 8057–8059; (d) R. Nagata, H. Yamanaka, E. Okazaki, I. Saito, Tetrahedron Lett. 1989, 30, 4995–4998; (c) R. Nagata, H. Yamanaka, E. Murahashi, I. Saito, Tetrahedron Lett. 1990, 31, 2907–2910.
- 2(a) M. E. Maier, Synlet 1995, 13–26; (b) K. C. Nicolaou, P. Maligres, J. Shin, E. de Leon, D. Rideout, J. Am. Chem. Soc. 1990, 112, 7825–7826; (c) K. C. Nicolaou, G. Skokotas, P. Maligres, G. Zuccarello, E. J. Schweiger, K. Toshima, S. Wendeborn, Angew. Chem. 1989, 101, 1255–1257; Angew. Chem. Int. Ed. Engl. 1989, 28, 1272–1275; (d) K. C. Nicolaou, W. M. Dai, Angew. Chem. Int. Ed. Engl. 1991, 103, 1453–1481 and 1991, 30, 1387–1416.
- 3(a)
Y. W. Andemichael,
Y. G. Gu,
K. K. Wang,
J. Org. Chem.
1992,
57, 794–796;
(b)
J. W. Grissom,
B. J. Slattery,
Tetrahedron Lett.
1994,
35, 5137–5410;
(c)
J. W. Grissom,
D. Huang,
J. Org. Chem.
1994,
59, 5114–5116;
(d)
Angew. Chem.
1995,
107, 2196–2198;
10.1002/ange.19951071825 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 2037–2039.
- 4 Even enyne ketenes undergo a reaction analogous to the Myers-Saito cyclization: (a) K. Nakatani, S. Isoe, S. Maekawa, I. Saito, Tetrahedron Lett. 1994, 35, 605–608; (b) H. Xia, H. W. Moore, J. Org. Chem. 1992, 57, 3765–3766; (c) A. Padwa, D. J. Austin, U. Chiacchio, J. M. Kassir, A. Rescifina, S. L. Xu, Tetrahedron Lett. 1991, 32, 5923–5926.
- 5 M. Schmittel, M. Strittmatter, S. Kiau, Tetrahedron Lett. 1995, 36, 4975–4979.
- 6 M. Schmittel, M. Strittmatter, K. Vollmann, S. Kiau, Tetrahedron Lett. 1996, 37, 999–1002.
- 7 J. E. Bennett, J. A. Howard, Chem. Phys. Lett. 1971, 9, 460–462.
- 8 The C1–C6 and C2–C6 distances determined by AM1 calculations (cf. M. J. S. Dewar, E. G. Zoebisch, E. F. Healy, J. J. P. Stewart, J. Am. Chem. Soc. 1985, 107, 3902–3909) for 1a in the s- cis conformation are 3.47 and 2.98 Å, respectively.
- 9 W. Carruthers, Cycloaddition Reactions in Organic Synthesis, Pergamon, Oxford 1990.
- 10(a) R. Brückner, R. Huisgen, J. Schmid, Tetrahedron Lett. 1990, 31, 7129–7132; (b) J. Sauer, R. Sustmann, Angew. Chem. 1980, 92, 773–801; Angew. Chem. Int. Ed. Engl. 1980, 19, 779–807.
- 11(a) M. Schmittel, C. Rüchardt, J. Am. Chem. Soc. 1987, 109, 2750–2759; (b) M. A. Flamm-ter Meer, H.-D. Beckhaus, K. Peters, H.-G. von Schnering, C. Rüchardt, Chem. Ber. 1985, 118, 4665–4673.
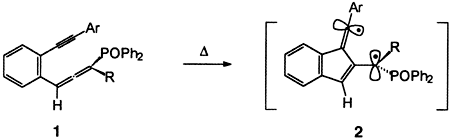
- 12 All enyne allenes described in this paper were prepared from the corresponding propargyl alcohols by rearrangement in the presence of chlorodiphenylphosphane. All starting materials and products were isolated as pure compounds and fully characterized by 1H, 13C, and 31P NMR spectroscopy, mass spectrometry, and IR spectroscopy (see Table 2).
- 13 R. Hollis, L. Hughes, V. W. Bowry, K. U. Ingold, J. Org. Chem. 1992, 57, 4284–4287.
- 14 The relative configuration is determined by the three contiguous sterogenic units: the stereogenic axis of the allene and the stereogenic centers in the cyclopropyl ring, C*H and C*HPh.
- 15(a) J. J. P. Stewart, J. Comput. Chem. 1989, 10, 209–221; (b) J. Comput. Aided Mol. Design 1990, 4, 1–105.
- 16 D. P. Curran, W. Shen, J. Am. Chem. Soc. 1993, 115, 6051–6059.
- 17(a) Since our first report on a C2–C6 cyclization two other papers have been published [17b, c] that report on a similar reaction leading to benzofulvene derivatives. However, in these cases owing to the substitution patterns a zwitterionic intermediate seems to be more plausible than a biradical. (b) T. Gillmann, T. Hülsen, W. Massa, S. Wocadlo, Synlet 1995, 1257–1259; (c) J. G. Garcia, B. Ramos, L. M. Pratt, A. Rodriguez, Tetrahedron Lett. 1995, 36, 7391–7394.