Structural Reorganization of the Doubly Protonated [222] Cryptand through Cation–π and Charge–Charge Interactions: Synthesis and Structure of Its [CoCl4]·0.5 C6H5CH3 Salt†
Leonard R. MacGillivray
Department of Chemistry University of Missouri-Columbia Columbia, MO 65211 (USA) Fax: Int. code +(573)884-9606 e-mail: [email protected]
Search for more papers by this authorCorresponding Author
Prof. Jerry L. Atwood
Department of Chemistry University of Missouri-Columbia Columbia, MO 65211 (USA) Fax: Int. code +(573)884-9606 e-mail: [email protected]
Department of Chemistry University of Missouri-Columbia Columbia, MO 65211 (USA) Fax: Int. code +(573)884-9606 e-mail: [email protected]Search for more papers by this authorLeonard R. MacGillivray
Department of Chemistry University of Missouri-Columbia Columbia, MO 65211 (USA) Fax: Int. code +(573)884-9606 e-mail: [email protected]
Search for more papers by this authorCorresponding Author
Prof. Jerry L. Atwood
Department of Chemistry University of Missouri-Columbia Columbia, MO 65211 (USA) Fax: Int. code +(573)884-9606 e-mail: [email protected]
Department of Chemistry University of Missouri-Columbia Columbia, MO 65211 (USA) Fax: Int. code +(573)884-9606 e-mail: [email protected]Search for more papers by this authorWe are grateful for funding from the National Science Foundation, the Natural Sciences and Engineering Research Council of Canada (NSERC) (research fellowship for L. R. M.), and the International Centre for Diffraction Data (research scholarship for L. R. M.).
Graphical Abstract
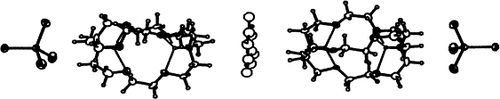
From an arene-rich liquid clathrate medium the title compound can be isolated in crystalline form. It is the first example of a compound in which an ionophore is stabilized by NH – π interaction. The crystal contains a linear supramolecular complex comprising five components (see picture) in which the cryptate molecules are elongated along their long axes.
– π interaction. The crystal contains a linear supramolecular complex comprising five components (see picture) in which the cryptate molecules are elongated along their long axes.
References
- 1 G. Gokel in Crown Ethers & Cryptands (Ed. J. F. Stoddart). Black Bear Press, Cambridge, 1991.
- 2 M. Dobler, Ionophores and Their Structures, Wiley, New York, 1981.
- 3 Lead references: (a) R. M. Izatt, K. Pawlak, J. S. Bradshaw, R. L. Bruening, Chem. Rev. 1991, 91, 1721; (b) L. Troxler, G. J. Wipff, J. Am. Chem. Soc. 1994, 116, 1468; (c) S. Lee, T. Wyttenbach, G. Von Helden, M. T. Bowers, J. Am. Chem. Soc. 1995, 117, 10159; (d) G. Papoyan, K. Gu, J. Wiorkiewicz-Kuczera, K. Kuczera, K. Bowman-James, J. Am. Chem. Soc. 1996, 118, 1354.
- 4(a) D. A. Dougherty, Science 1996, 271, 163; (b) R. A. Kumpf, D. A. Dougherty, Science 1993, 261, 1708; (c) J. W. Caldwell, P. A. Kollman, J. Am. Chem. Soc. 1995, 117, 4177.
- 5(a) J. Gross, G. Harder, F. Vögtle, H. Stephan, K. Gloe, Angew. Chem. 1995, 107, 523; Angew. Chem. Int. Ed. Engl. 1995, 34, 481; (b) P. C. Kearney, L. S. Mizoue, R. A. Kumpf, J. E. Forman, A. McCurdy, D. A. Dougherty, J. Am. Chem. Soc. 1993, 115, 9907; (c) M. A. Petti, T. J. Shepodd, R. E. Barrans, Jr., D. A. Dougherty, J. Am. Chem. Soc. 1988, 110, 6825.
- 6 D. A. Dougherty, D. A. Stauffer, Science 1990, 250, 1558.
- 7(a) M. Meot-Ner (Mautner), C. A. Deakyne, J. Am. Chem. Soc. 1985, 107, 469; (b) C. A. Deakyne, M. Meot-Ner (Mautner), J. Am. Chem. Soc. 1985, 107, 474.
- 8 J. Novotny, R. E. Bruccoleri, F. A. Saul, Biochemistry, 1989, 28, 4735.
- 9 J. L. Atwood in Separation Technology (Eds.: N. N. Li, H. Strathmann), United Engineering Trustees, New York, 1988, pp. 46–56.
- 10(a) L. R. MacGillivray, J. L. Atwood, J. Chem. Soc. Chem. Commun. 1996, 735; (b) J. Org. Chem. 1995, 60, 4972; (c) P. C. Junk, J. L. Atwood, J. Chem. Soc. Chem. Commun. 1995, 1552; (d) P. C. Junk, L. R. MacGillivray, M. T. May, K. D. Robinson, J. L. Atwood, Inorg. Chem. 1995, 34, 5395; (e) J. L. Atwood, S. G. Bott, A. W. Coleman, K. D. Robinson, S. B. Whetstone, C. M. Means, J. Am. Chem. Soc. 1987, 109, 8100.
- 11 Crystal data for [1-2H][CoCl4]·0.5C6H5CH3: crystal size: 0.10 × 0.20 × 0.20 mm, monoclinic, space group C2/c, a = 19.478(7), b = 11.245(1), c = 26.43(1) Å, β = 104.24(2), V = 5611(3) Å3, ρcalcd = 1.48 gcm−3, 2θmax = 46°; μ = 0.52 mm−1, MoKα radiation (λ = 0.71069 Å) for Z = 8. Intensity data were collected using the ω–2θ scan mode on an Enraf Nonius CAD-4 diffractometer and were corrected for Lorentz, polarization, and absorption effects, but not for extinction. The structure was solved with direct methods. Least-squares refinement based on 1301 reflections with Inet > 2.0σ(Inet) (out of 4043 unique reflections), and 298 parameters on convergence gave final values of R = 0.081 and Rw = 0.065. Methylene and aromatic hydrogen atoms were placed in calculated positions (d(C,H) = 1.08 and 1.00 Å, respectively) with their positional and thermal parameters fixed, temperature factors being based upon the carbon atoms to which they are bonded. Quaternary ammonium hydrogen atoms were located by inspection of a difference Fourier map, and their positional and thermal were fixed, temperature factors being based upon the atoms to which they are bonded. The toluene molecule was observed to lie on a crystallographic twofold axis and exhibit threefold disorder with the carbon atoms of the methyl group displaying site occupancies of 0.4 (CS5) and 0.3 (CS5B). Crystallographic calculations were conducted with the NRCVAX program package (E. J. Gabe, Y. Le Page, J.-P. Charland, F. L. Lee, P. S. White, J. Appl. Cryst. 1989, 22, 384) on a pentium-based IBM compatible computer. Crystallographic data (excluding structure factors) for the structure reported in this paper have been desposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-179-83. Copies of the data can be obtained free of charge on application to The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: Int. +(1223)336-033; e-mail: [email protected]).
- 12 The 1H NMR spectrum exhibits resonances typical of the [1-2H]2+ ion (see ref. [10b]) and toluene.
- 13 Satisfactory elemental analysis was obtained for 2 as formulated.
- 14 C. B. Aakeröy, K. R. Seddon, Chem Soc. Rev 1993, 397.
- 15 B. Metz, D. Moras, R. Weiss, J. Chem. Soc. Perkin Trans. 2 1976, 423.
- 16 The N ··· N separation in 2 is also comparable to that in [H3O][1-2H] [(CoCl3)2(μ-Cl)] (6.44 Å) [10a] in which two strong interionic O+-H ··· O hydrogen bonds cause reorganization of the cryptate and result in the formation of two bifurcated intraionic hydrogen bonds within the cavity.
- 17 M. A. Viswamitra, R. Radhakrishnan, J. Bandekar, G. R. Desiraju, J. Am. Chem. Soc. 1993, 115, 4868.
- 18
Ab initio calculations involving the N(CH3)
 ion confirm the electrostatic nature of such interactions and demonstrate that a conformation in which three hydrogen atoms are attached to three different alkyl groups is preferred [7].
ion confirm the electrostatic nature of such interactions and demonstrate that a conformation in which three hydrogen atoms are attached to three different alkyl groups is preferred [7].
- 19 R. Hunter, R. H. Haueisen, A. Irving, Angew. Chem. 1994, 106, 588; Angew. Chem. Int. Ed. Engl. 1994, 33, 566.
- 20 L. R. Hanton, C. A. Hunter, D. H. Purvis, J. Chem. Soc. Chem. Commun. 1992, 1134.
- 21 We also note the following data: (a) an interionic Cl ··· Cl separation of 3.465(8) Å and a Co-Cl-Cl angle of 179.6(2)° between Cl4 and Cl4a (a: 1.5 - x, 0.5 - y, - z), which is indicative of a Cl ··· Cl interaction [see V. R. Pedireddi, D. S. Reddy, B. S. Goud, D. C. Craig, A. D. Rae, G. R. Desiraju, J. Chem. Soc. Perkin Trans. 1994, 2354]; and (b) close approach between the methyl group of the disordered toluene molecule and the [CoCl4]2− ion (CMe ··· Cl < 3.60 Å), which suggests the presence of a stabilizing interaction between the hydrogen atoms of the substituent and the anion.
- 22 J. L. Atwood, J. Inclusion Phenom. 1985, 3, 13.
- 23 Note added in proof (August 1, 1996): Using data collected on the Siemens SMART system we have recently discovered that 2 may also be solved in the trigonal space group R3c with a = 11.2733(5), c = 77.002(5) Å. The dication and anion lie on a crystallographic threefold axis and the toluene molecule exhibits threefold disorder across a crystallographic 32 site. Interpretation of the assembly process, however, remains the same.