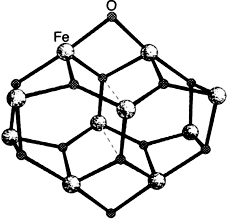
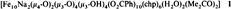Ferric Wheels and Cages: Decanuclear Iron Complexes with Carboxylato and Pyridonato Ligands†
Dr. Simon Parsons
Department of Chemistry, The University of Edinburgh West Mains Road, Edinburgh, EH9 3JJ (UK) Fax: Int. code +(131)667-4743
Search for more papers by this authorDr. Gregory A. Solan
Department of Chemistry, The University of Edinburgh West Mains Road, Edinburgh, EH9 3JJ (UK) Fax: Int. code +(131)667-4743
Search for more papers by this authorCorresponding Author
Dr. Richard E. P. Winpenny
Department of Chemistry, The University of Edinburgh West Mains Road, Edinburgh, EH9 3JJ (UK) Fax: Int. code +(131)667-4743
Department of Chemistry, The University of Edinburgh West Mains Road, Edinburgh, EH9 3JJ (UK) Fax: Int. code +(131)667-4743Search for more papers by this authorProf. Cristiano Benelli
Dipartimento di Chimica, Universita degli Studi di Firenze (Italy)
Search for more papers by this authorDr. Simon Parsons
Department of Chemistry, The University of Edinburgh West Mains Road, Edinburgh, EH9 3JJ (UK) Fax: Int. code +(131)667-4743
Search for more papers by this authorDr. Gregory A. Solan
Department of Chemistry, The University of Edinburgh West Mains Road, Edinburgh, EH9 3JJ (UK) Fax: Int. code +(131)667-4743
Search for more papers by this authorCorresponding Author
Dr. Richard E. P. Winpenny
Department of Chemistry, The University of Edinburgh West Mains Road, Edinburgh, EH9 3JJ (UK) Fax: Int. code +(131)667-4743
Department of Chemistry, The University of Edinburgh West Mains Road, Edinburgh, EH9 3JJ (UK) Fax: Int. code +(131)667-4743Search for more papers by this authorProf. Cristiano Benelli
Dipartimento di Chimica, Universita degli Studi di Firenze (Italy)
Search for more papers by this authorThis work was supported by the Engineering and Physical Sciences Research Council and the Leverhulme Trust.
Graphical Abstract
References
- 1(a) R. Sessoli, D. Gatteschi, A. Caneschi, M. A. Novak, Nature 1993, 365, 141; (b) H. J. Eppley, H.-L. Tsai, N. de Vries, K. Folting, G. Christou, D. N. Hendrickson, J. Am. Chem. Soc. 1995, 117, 301.
- 2 D. P. Goldberg, A. Caneschi, S. J. Lippard, J. Am. Chem. Soc. 1993, 115, 9299.
- 3 M. W. Wemple, D. Adams, K. S. Hagen, K. Folting, D. N. Hendrickson, G. Christou, J. Chem. Soc. Chem. Commun. 1995, 1591.
- 4 A. J. Blake, C. M. Grant, C. I. Gregory, S. Parsons, J. M. Rawson, D. Reed, R. E. P. Winpenny, J. Chem. Soc. Dalton Trans. 1995, 195.
- 5 A. J. Blake, C. M. Grant, S. Parsons, J. M. Rawson, R. E. P. Winpenny, J. Chem. Soc. Chem. Commun. 1994, 2363.
- 6 A. J. Blake, E. K. Brechin, A. Codron, R. O. Gould, C. M. Grant, J. M. Rawson, R. E. P. Winpenny, J. Chem. Soc. Chem. Commun. 1995, 1983.
- 7 Crystal data for 1·2 THF (C48H106Fe10O42): monoclinic, P21/n, a = 9.027(3), b = 22.101(8), c = 20.159(12) Å, β = 102.54(3)°, V = 3926 Å3, M = 1914, Z = 2 (the molecule lies on an inversion center), ρcalcd = 1.619 g cm−3, T = 150.0(2) K, crystal size 0.35 × 0.15 × 0.05 mm, μ(MoKα) = 1.879 mm−1. Crystal data for 3·8 MeCN·2 Me2CO (C128H124Cl6Fe10N14Na2O42): triclinic, P1, a = 16.195(12), b = 17.604(14), c = 17.593(14) Å, α = 61.48(5), β = 63.18(5), γ = 62.72(5)°, V = 3740 Å3, M = 3347, Z = 1 (the molecule lies on an inversion center), ρcalcd = 1.487 g cm−3, T = 150.0(2) K, crystal size 0.78 × 0.58 × 0.35 mm, μ(MoKα) = 1.134 mm−1. Data were collected with a Stoe-Stadi-4 diffractometer, graphite monochromator, MoKα, ω–2θ scans with on-line profile-fitting (W. Clegg, Acta Crystallogr. Sect. A 1981, 37, 22). An absorption correction was applied using ω-scan data for 3 (min./max. transmission 0.392, 0.560). For both 1 and 3 the crystals were cooled using an Oxford Cryosystems low-temperature device (J. Cosier, A. M. Glazer, J. Appl. Crystallogr. 1986, 19, 105). Both structures were solved by direct methods and completed by iterative cycles of difference Fourier syntheses and full-matrix least-squares refinement against F2 (G. M. Sheldrick, SHELXL-93, University of Göttingen, 1993). Hydrogen atoms were included in both structures in calculated positions, riding on parent C atoms, with U(H) = 1.2 Ueq(C) for chp H atoms and U(H) = 1.5 Ueq(C) for methyl H atoms. For 1 four bridging MeO groups are disordered, and one molecule of THF solvate is also disordered over two positions and was refined with similarity restraints. All iron and fully weighted oxygen atoms (with the exception of O10 which is part of the THF solvate) were refined with anisotropic displacement parameters to give, using 349 parameters, wR2 = 0.2357 for 3651 unique data (2θ ≤ 40°) [R1 = 0.0941 for 1820 observed reflections, F0 > 4σ(F)]. The largest residual difference peak and hole were 0.977 and −0.749 eÅ−3, respectively. For 3 all non-hydrogen atoms were refined anisotropically, except atoms in several disordered solvent molecules, which were refined isotropically, to give, using 864 parameters, wR2 = 0.2236 for 13157 unique data (2θ ≤ 50°) [R1 = 0.0798 for 7629 observed reflections, F0 > 4σ(F)]. The largest residual difference peak and hole were 1.472 and −1.349 eÅ−3, Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-179-75. Copies of the data can be obtained free of charge on application to The Director, CCDC, 12 Union Road, Cambridge CB21EZ, UK (fax: Int. code +(1223)336-033; e-mail: [email protected]).
- 8 K. L. Taft, C. D. Delfs, G. C. Papefthymiou, S. Foner, D. Gatteschi, S. Lippard, J. Am. Chem. Soc. 1994, 116, 823.
- 9 W. Armstrong, S. J. Lippard, Inorg. Chem. 1985, 24, 981.
- 10 S. Parsons, G. A. Solan, R. E. P. Winpenny, J. Chem. Soc. Chem. Commun. 1995, 1987.
- 11 A. J. Blake, S. Parsons, G. A. Solan, R. E. P. Winpenny, J. Chem. Soc. Dalton Trans. 1996, 321.
- 12 The related dinuclear complex [Fe2O(O2CPh)2(chp)2(phen)2], which we have structurally characterized, can be isolated by addition of 1,10-phenanthroline (phen). S. Parsons, G. A. Solan, R. E. P. Winpenny, unpublished results.
- 13 K. L. Taft, G. C. Papaefthymiou, S. J. Lippard, Science 1993, 259, 1302.
- 14 W. Micklitz, S. J. Lippard, J. Am. Chem. Soc. 1989, 111, 6856.
- 15 A. K. Powell, S. L. Heath, D. Gatteschi, L. Pardi, R. Sessoli, G. Spina, F. Del Giallo, F. Pieralli, J. Am. Chem. Soc. 1995, 117, 2491.
- 16 D. M. Kurtz, Jr., Chem. Rev. 1990, 90, 589.
- 17 A. Earnshaw, B. N. Figgis, J. Lewis, J. Chem. Soc. A 1966, 1656.