Journal list menu
Export Citations
Download PDFs
Cover Picture (Angew. Chem. Int. Ed. Engl. 4/1986)
- First Published: April 1986
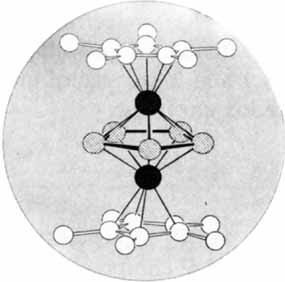
The cover shows the structure of a complex in which, for the first time, cyclo-P5 could be stabilized. The planar cyclo-P5 forms the middle layer of a tripledecker complex, which is completed by two chromium atoms and two pentamethyecyclopentadienyl ligands. Since a cyclo-P6 complex (“hexaphosphabenzene”) was synthesized last year by the same research group and since cyclo-P4 (“tetraphosphabutadine”) also be stabilized in a complex? Further details on the cyclo-P5 complex are reported by O. J. Scherer et al. on page 363ff.
Graphical Abstract (Angew. Chem. Int. Ed. Engl. 4/1986)
- First Published: April 1986
Reviews
Adolf von Baeyer's Scientific Achievements — a Legacy†
- Pages: 297-311
- First Published: April 1986

A fascinating episode in the history of organic chemistry comes alive through examination of Adolf von Baeyer's work. Not only the ring-strain theory, which is now one hundred years old, but also numerous discoveries concerning indigo, benzene, and many other classes of substances are linked with Baeyer's name. The picture on the right shows the scientist at 72 years of age.
The Concept of Strain in Organic Chemistry
- Pages: 312-322
- First Published: April 1986
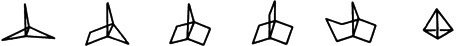
The ring-strain theory, which Adolf von Baeyer formulated one hundred years ago, has been expanded in many directions; today, strain is discussed in terms of bond-length and bond-angle distortions as well as nonbonding interactions. Only in such terms can the stability of such highly strained compounds as tetra-tert-butyltetrahedrane and [1.1.1]propellane be understood.
Ordering of Ionic Solutes in Dilute Solutions through Attraction of Similarly Charged Solutes—A Change of Paradigm in Colloid and Polymer Chemistry†‡
- Pages: 323-334
- First Published: April 1986
The crucial role of counterions in solutions of macroions and ionic latex particles is revealed by a new theory, which explains the surprising behavior of these species. According to X-ray spectroscopic studies, these ions are arranged nearly regularly—lattice-like—in dilute solutions. Measured and calculated distances can be interpreted in terms of attraction between similarly charged particles in the presence of counterions.
Communications
10,10′-Bi(trispiro[2.0.2.0.2.1]decylidene): a Highly Nucleophilic Olefinic Hydrocarbon†‡
- Pages: 335-336
- First Published: April 1986
![10,10′-Bi(trispiro[2.0.2.0.2.1]decylidene): a Highly Nucleophilic Olefinic Hydrocarbon](/cms/asset/a5be4c58-f66f-4891-a551-cea692a6f42e/must001.jpg)
The title compound 1 is a record olefin in more ways than one. Not only the four α-but also the two β-spirocyclopropane groups exert a donor effect on the double bond. The π-ionization potential of 7.3 eV and the oxidation potential of 0.74 V are the lowest values observed so far for monoolefins without heteroatom donor substituents.
Bicyclic Host Cavities Derived from Triphenylamine—Guest Selectivity and Redox Properties†‡
- Pages: 336-338
- First Published: April 1986
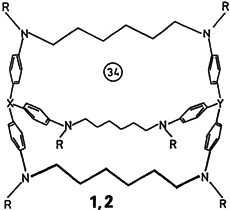
Large, substrate-selective cavities are present in the new, water-soluble macrobicyclic compounds 1, X, Y N, and 2, X N, Y C-CH3. Because the triphenylamine nitrogen can be oxidized electrochemically to the radical cation, these host compounds may be electrochemically modified (“switched on and off”). Compounds such as 1 and 2 are also of interest as models for redox enzymes.
[(η5-C5H5)2Mo2(CO)4(PPh)2], a Diphosphene Complex with a Butterfly Structure†
- Pages: 338-339
- First Published: April 1986
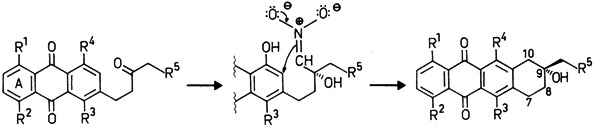
Simple Synthesis of Anthracyclinones by Cyclization of an Intermediate Hydroxynitronate†
- Pages: 339-340
- First Published: April 1986
Structure Determinations of Pentatetraenes—Comparison of the Structures of Cumulenes†‡
- Pages: 340-342
- First Published: April 1986
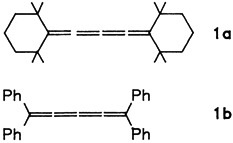
The number (n) of double bonds in a cumulene system has a very strong effect on the structure. For cumulenes with n = 3 and 5, both the alternation in the bond lengths and the substituent effects are larger than for allenes (n = 2) and cumulenes with n = 4. The structure analyses of 1a and 1b have now made possible the direct comparison of variously substituted pentatetraenes.
The Elucidation of the Constitution of Glycopeptides by the NMR Spectroscopic COLOC Technique
- Pages: 342-344
- First Published: April 1986
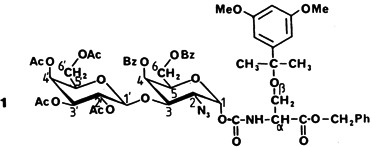
The structure elucidation of the disaccharide-serine derivative 1 demonstrates how the two-dimensional NMR spectroscopic COLOC technique may be used to assign heteronuclear long-range couplings and thus answer the following questions: (1) How are the sugar residues linked? (2) Is the sugar indeed linked to the amino acid through a urethane unit? (3) At which positions are the various protecting groups?
The Phenol ⇌ 2,4-Cyclohexadienone Equilibrium in Aqueous Solution†
- Pages: 344-345
- First Published: April 1986
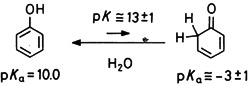
The “aromaticity” of phenol is manifested by an increase in the enolization constant and the CH acidity constant of 2,4-cyclohexadienone by more than 20 orders of magnitude compared with the corresponding constants of acetone. The first experimental estimate of these values was achieved by the combination of kinetic measurements with flash photolysis and 1H-NMR spectroscopy.
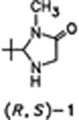
Enantiomer Separation of (R,S)-2-(tert-Butyl)-3-methyl-4-imidazolidinone, a Chiral Building Block for Amino Acid Synthesis†
- Pages: 345-346
- First Published: April 1986
Organic Reactions at High Pressure: Cycloaddition Reactions of 11-Methylene-1,6-methano[10]annulene†
- Pages: 346-348
- First Published: April 1986
![Organic Reactions at High Pressure: Cycloaddition Reactions of 11-Methylene-1,6-methano[10]annulene](/cms/asset/3866a586-179c-460c-a33a-f00e8e1f7c14/must001.jpg)
A remarkable pressure dependence is exhibited by the reaction of 11-methylene-1,6-methano[10]annulene with dicyanoacetylene. At normal pressure, one obtains the 1:1 adduct 1a/1b, a fluxional system that is the first example of its kind of a methylenenorcaradiene/heptafulvene valence tautomerism. At 7000 bar, the main product formed is the 2:1 adduct 2. Despite the less favorable geometry, 2 must have been formed by [8+2] cycloaddition of dicyanoacetylene to 1b.
Tetrakis(diphenylphosphino)allene†
- Pages: 348-349
- First Published: April 1986
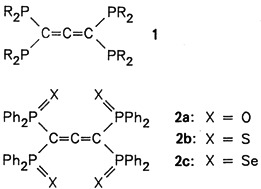
A one-pot reaction afforded the title compound 1, R Ph, in 60% yield via lithiation of 1-diphenylphosphinopropyne with n BuLi followed by reaction with ClPPh2. The colorless, crystalline allene 1 is very reactive: for example, it reacts with oxygen, sulfur, and selenium to give the corresponding chalcogenides 2a-c.
First Crystal Structure Analysis of an Aliphatic Carbocation—Stabilization of the 3,5,7-Trimethyl-1-adamantyl Cation by CC Hyperconjugation†
- Pages: 349-350
- First Published: April 1986
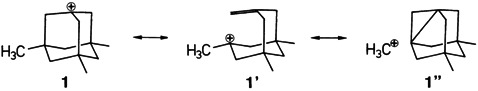
That hyperconjugation can result in extreme stabilization is confirmed by the first X-ray structure analysis of an aliphatic carbocation. Until now, only carbocations stabilized by π-electron systems or heteroatoms had been studied. The differences in bond lengths in the 3,5,7-trimethyladamantyl cation 1 (0.18(2) Å) are greater than those in compounds exhibiting the largest anomeric effects. 1′ is therefore the best description of the structure of the cation.
Ge4.06I, an Unexpected Germanium Subiodide—a Tetragermanioiodonium(III) Iodide with Clathrate Structure [Ge46 − xIx]I8, x = 8/3
- Pages: 350-352
- First Published: April 1986
![Ge4.06I, an Unexpected Germanium Subiodide—a Tetragermanioiodonium(III) Iodide with Clathrate Structure [Ge46 − xIx]I8, x = 8/3](/cms/asset/33fa290e-9f6a-4b88-9f70-232ff989d0e4/mgra001.jpg)
Upon rapid thermal decomposition of GeI2—an important reaction for the chemical transport of germanium—the metastable subiodide Ge4.06I, a semiconductor with a clathrate structure, is formed. The clathrate framework is made up of Ge and I3+, and I− ions are present in the cavities: [Ge46-8/3I8/3]I8·Ge4.06I forms dendrite-like intergrown cubes, as shown in the picture on the right.
Valence Tautomerism of the Heptaheteronortricycles [P7 − xAsx]3−†
- Pages: 352-353
- First Published: April 1986
Solutions of the mixed crystals Rb3[P7−xAsx] in ethylenediamine contain almost the entire series of the heptaheteronortricycle anions P7−xAsx, as has been demonstrated by 31P-NMR spectroscopy; only [PAs6]3- could not be detected with certainty. [P6As]3− and [P5As2]3− are preferentially formed, but not [P4As3]3− as would be expected on the basis of the concept of topological charge stabilization. Very suprisingly, exchange reactions do not take place.
[NbAs8]3−, a Novel Type of Complex and an Unexpected One-Dimensional Chain Structure:  [Rb{NbAs8}]2−
[Rb{NbAs8}]2−
- Pages: 353-354
- First Published: April 1986
![[NbAs8]3−, a Novel Type of Complex and an Unexpected One-Dimensional Chain Structure: [Rb{NbAs8}]2−](/cms/asset/21c9823a-243f-44c3-92ca-d9360253d566/must001.jpg)
A serendipitous result was obtained when too much rubidium was used in the synthesis of Rb3As7 in a niobium tube: the surprising product is a complex containing the anion [NbAs8]3− 1. This is the first example of an octacycle acting as an η8-ligand to coordinate a metal atom at its center. With Rb+, one-dimensional chains 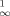 [Rb{NbAs8}]2− are formed, even though the strong complex formers 2,2,2-crypt and ethylenediamine were present during the crystallization.
[Rb{NbAs8}]2− are formed, even though the strong complex formers 2,2,2-crypt and ethylenediamine were present during the crystallization.
Intramolecular Addition of 2-(Trimethylsilyl)methyl-substituted Alkyl Cations to Double Bonds–Synthesis of Bicyclo[3.3.0]octane Derivatives†‡
- Pages: 354-356
- First Published: April 1986
![Intramolecular Addition of 2-(Trimethylsilyl)methyl-substituted Alkyl Cations to Double Bonds–Synthesis of Bicyclo[3.3.0]octane Derivatives](/cms/asset/9d1575cd-5f0c-418f-8a7b-8239b5a0c6e3/must001.jpg)
The title reaction, a transformation that theoretically should be difficult, has been successfully carried out. The exploitation of two “β effects” was crucial: β-silicon stabilizes the intermediate allyl cation and two geminal methyl groups in the β position control the course of the reaction. An example is the transformation of 1 into 2, which is converted into 3 under acid catalysis.
An α-Functionalized Organomanganese(II) Derivative: a Dinuclear Manganese(II) Complex with a σ-Bond α-Thioalky Ligand†
- Pages: 356-357
- First Published: April 1986
The coordination number 5, two MnC σ-bonds and two hard donor ligands characterize the organomanganese(II) complex 1. It is accessible by reaction of [MnCl2(thf)2] with phenylthiomethyllithium and is stable at room temperature (X-ray structure analysis). Compound 1 reacts like a typical Grignard reagent with benzaldehyde.
η3-1-Phosphaallyliron Complexes with Additional W(CO)5-Coordination at the Phosphorus—the 1-Phosphaallyl Ligand as 5e-Donor
- Pages: 357-359
- First Published: April 1986
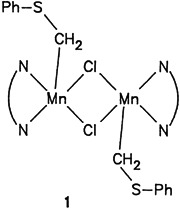
Binuclear WFe complexes containing a phosphaallyl bridge can be synthesized from W(CO)5-stabilized vinylphosphanes such as 1 (R H) by reaction with the Fe2 complex 2. In the product 3, the bridging ligand functions simultaneously as a 2e and 3e donor. The structure was elucidated by X-ray diffraction analysis.
The Schrock Carbyne-Complex [Cl3(dme)WCCMe3], a Highly Reactive Catalyst for the Metathesis of Alkenes†
- Pages: 359-360
- First Published: April 1986
![The Schrock Carbyne-Complex [Cl3(dme)WCCMe3], a Highly Reactive Catalyst for the Metathesis of Alkenes](/cms/asset/a95af27e-939e-48fc-a33f-b5707b191926/must001.jpg)
A metallacycle that is both an alkyl and a carbene complex is the active catalyst in the metathesis of cyclopentene and 1-octene with [Cl3(dme)WCCMe3] (dme = dimethoxyethane). Poly-1-pentenylene (70%) is formed from cyclopentene in 3 h at 20°C; complexes such as 1 are assumed to be key intermediates. These results explain, in particular, the steric course of metathesis reactions.
Synthesis of O-Glycopeptides of the Tumor-Associated TN- and T-Antigen Type and Their Binding to Bovine Serum Albumin†‡
- Pages: 360-362
- First Published: April 1986
The synthesis of immunologically relevant conjugates from bovine serum albumin (BSA) and glycotripeptides with TN- and T-antigen structures has been achieved for the first time. In the glycoserylserylthreonines, two monosacharide (TN) and two disaccharide (T) units are α-glycosidically linked with serine and threonine; they correspond to the N-terminal structural elements of glycophorin A with M blood group characteristics. The synthesis is accomplished by means of a special protecting group technique (Sacch = mono- or disaccharide unit).
cyclo-P5 as Complex Ligand—the Phosphorus Analogue of the Cyclopentadienyl Ligand†‡
- Pages: 363-364
- First Published: April 1986
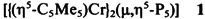
A milestone for the chemistry of phosphorus-containing complexes is the synthesis of the black-red cyclo-P5 complex 1, which is metallic in appearance. In light of the overwhelming significance of the cyclopentadienyl ligand in organometallic chemistry, the isovalent isoelectronic cyclo-P5 would appear to hold much promise. ESR investigations indicate that 1 is a mixed valence complex (d4/d5 system), which can be readily oxidized and reduced.
Reductive Coupling of CO: Formation of a 1:1 Adduct of η2-Ketone- and Enediolato-Complex upon Carbonylation of Bis(cyclopentadienyl)hafnacyclobutane†‡
- Pages: 364-365
- First Published: April 1986
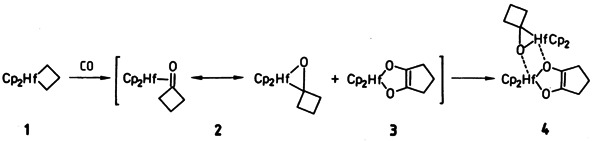
The hafnacyclobutane 1 takes up 1.5 equivalents of CO very rapidly even under normal conditions, forming the polycyclic complex 4 as sole product in good yield. 4 is a stable 1:1 adduct of [(η2-ccyclobutanone)HfCp2] 2 and the hafnocene enediolate complex 3, the formation of which involves the coupling of two CO molecules——possibly via the intermediacy of the η2-ketone complex 2.
Methylmalonylcarba(dethia)-Coenzyme A as Substrate of the Coenzyme B12-Dependent Methylmalonyl-CoA Mutase: Enzymatic Rearrangement of a β- to a γ-Keto Acid†‡
- Pages: 366-367
- First Published: April 1986
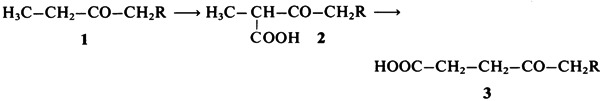
Coenzyme A analogues with CH2 instead of S are model compounds that may be used to gain insight into the mechanism of action of methylmalonyl-CoA mutase. Propionylcarba(dethia)coenzyme A 1 was synthesized and enzymatically carboxylated to the methylmalonate derivative 2. The coenzyme B12-dependent enzymatic rearrangement of the β-keto acid 2 to the γ-keto acid 3 is the first of its kind to be described.
Nickel-Induced Cyclotetramerization of Cyclopropabenzene to 1,6:7,12:13,18:19,24-Tetrakismethano[24]annulene†‡
- Pages: 367-368
- First Published: April 1986
![Nickel-Induced Cyclotetramerization of Cyclopropabenzene to 1,6:7,12:13,18:19,24-Tetrakismethano[24]annulene](/cms/asset/1bbf4e88-a2e0-4e58-a31d-1c2e3768e83b/must001.jpg)
The [24]annulene 1 containing four CH2 bridges is surprisingly formed in the twostep organometallic title reaction. The key intermediate is a bismethano-bridged nickelacyclotridecahexaene with two PMe3 ligands. Upon treatment of this complex with PMe3, the final product 1 is formed by reductive elimination.
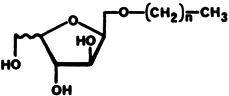
Carbohydrate-Based Liquid Crystals: Mesogenic 1-O-Alkyl Derivatives of 2,5-Anhydrohexitols†
- Pages: 368-369
- First Published: April 1986
[Cp(CO)2Mo{NR*[Rh(norbornadiene)]-CH(pyridyl)}], a Rhodium Complex with an Asymmetric Molybdenum Atom in the Chelate Skeleton†
- Pages: 371-373
- First Published: April 1986
![[Cp(CO)2Mo{NR*[Rh(norbornadiene)]-CH(pyridyl)}], a Rhodium Complex with an Asymmetric Molybdenum Atom in the Chelate Skeleton](/cms/asset/5675e935-cf92-48b0-90dd-404053f671f7/must001.jpg)
Four centers of chirality—2 × C, 1 × N, 1 × Mo—are present in the title compound 1, R* = (R)-1-CHMePh. Complex 1 is characterized, among other things, by a slightly bent CO group, which is in close contact to Rh. A complicated series of ring openings and epimerizations is suggested as a plausible route to 1.
[(α-Cyanobenzyllithium. Tetramethylethylenediamine)2. Benzene]: X-ray Structure Analysis of an α-Nitrile “Carbanion”†
- Pages: 373-374
- First Published: April 1986
![[(α-Cyanobenzyllithium. Tetramethylethylenediamine)2. Benzene]: X-ray Structure Analysis of an α-Nitrile “Carbanion”](/cms/asset/06aef2e5-faa4-42d5-863f-15f5d0763bc1/must001.jpg)
A “ketene iminate” and not a carbanion is present in the title compound 1·C6H6. The bond lengths in the CCN group deviate considerably from those in ketene imines such as 2. In comparison, enolates have practically the same bond lengths as enol ethers. This difference is due to the different stabilization of a negative charge by nitriles and carbonyl compounds.
Benzo[c]benzo[3,4]cinnolino[1,2-a]cinnoline, a Chiral Hydrazine Derivative†‡
- Pages: 374-375
- First Published: April 1986
![Benzo[c]benzo[3,4]cinnolino[1,2-a]cinnoline, a Chiral Hydrazine Derivative](/cms/asset/67acd759-bd99-4694-8bfd-68164747e0e5/must001.jpg)
The spontaneous crystallization of the enantiomers of the title compound 1 (space group P21) has been observed. The X-ray structure analysis reveals a pyramidal arrangement of the substituents on the nitrogen atoms. The large barrier to racemization (27.1 kcal mol−1) is largely due to the gauche effect of the vicinal lone pairs of electrons.
Strontium and Barium Alkoxostannates(II)—Molecules with S6 Symmetry†
- Pages: 375-377
- First Published: April 1986
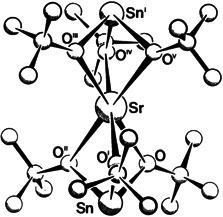
A quantitative separation of calcium and strontium is made possible by the reaction of M(OtBu)2 (M Sr, Ca) with [Sn(OtBu)2]2 in benzene (!). The strontium salt dissolves quantitatively; the calcium salt is insoluble. In the process, the polycycle shown on the right is formed; it is even soluble in hexane and its structure is reminiscent of that of SrO.
Diels-Alder Adducts of Benzene with Arenes and Their [4+2] Cycloreversion†
- Pages: 377-378
- First Published: April 1986
Book Reviews
Book Review: The Chemist's English. By Robert Schoenfeld
- Pages: 378-379
- First Published: April 1986
Book Review: Spectral Atlas of Polycyclic Aromatic Compounds. By W. Karcher, R. J. Fordham, J. J. Dubois, P. G. J. M. Claude, and J. A. M. Lighthart
- Pages: 379-380
- First Published: April 1986
Book Review: Biotechnology. A Comprehensive Treatise in 8 Volumes. Series editors: H.-J. Rehm, G. Reed. Vol. 6a: Biotransformations. K. Kieslich
- Page: 380
- First Published: April 1986
Book Review: Electroorganic Chemistry as a New Tool in Organic Synthesis. By T. Shono
- Pages: 380-381
- First Published: April 1986
Book Review: Synthetic Organic Photochemistry. Edited by W. M. Horspool
- Page: 381
- First Published: April 1986
Book Review: Techniques in Organic Reaction Kinetics. By P. Zuman and R. C. Patel
- Pages: 381-382
- First Published: April 1986




![[(η5-C5H5)2Mo2(CO)4(PPh)2], a Diphosphene Complex with a Butterfly Structure](/cms/asset/d9578803-dba6-4179-b17f-4adef8c7c03b/must001.jpg)

![(Z)-[6]Paracycloph-3-ene](/cms/asset/1e49bb00-3a6b-4cd3-baaa-f2cd197bdb7e/must001.jpg)
![Diels-Alder Adducts of Benzene with Arenes and Their [4+2] Cycloreversion](/cms/asset/562de2f3-9f14-453c-a7a1-0d9d4bccf442/must001.jpg)


































