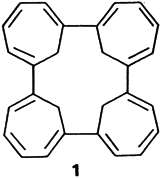
Nickel-Induced Cyclotetramerization of Cyclopropabenzene to 1,6:7,12:13,18:19,24-Tetrakismethano[24]annulene†‡
Dr. Richard Mynott
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-4330 Mülheim-Ruhr (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Richard Neidlein
Pharmazeutisch-chemisches Institut der Universität, Im Neuenheimer Feld 364, D-6900 Heidelberg (FRG)
Richard Neidlein, Pharmazeutisch-chemisches Institut der Universität, Im Neuenheimer Feld 364, D-6900 Heidelberg (FRG)
Günther Wilke, Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-4330 Mülheim-Ruhr (FRG)
Search for more papers by this authorDipl.-Chem. Harald Schwager
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-4330 Mülheim-Ruhr (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Günther Wilke
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-4330 Mülheim-Ruhr (FRG)
Richard Neidlein, Pharmazeutisch-chemisches Institut der Universität, Im Neuenheimer Feld 364, D-6900 Heidelberg (FRG)
Günther Wilke, Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-4330 Mülheim-Ruhr (FRG)
Search for more papers by this authorDr. Richard Mynott
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-4330 Mülheim-Ruhr (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Richard Neidlein
Pharmazeutisch-chemisches Institut der Universität, Im Neuenheimer Feld 364, D-6900 Heidelberg (FRG)
Richard Neidlein, Pharmazeutisch-chemisches Institut der Universität, Im Neuenheimer Feld 364, D-6900 Heidelberg (FRG)
Günther Wilke, Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-4330 Mülheim-Ruhr (FRG)
Search for more papers by this authorDipl.-Chem. Harald Schwager
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-4330 Mülheim-Ruhr (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Günther Wilke
Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-4330 Mülheim-Ruhr (FRG)
Richard Neidlein, Pharmazeutisch-chemisches Institut der Universität, Im Neuenheimer Feld 364, D-6900 Heidelberg (FRG)
Günther Wilke, Max-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, D-4330 Mülheim-Ruhr (FRG)
Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
Dedicated to Professor Heinz A. Staab on the occasion of his 60th birthday
Graphical Abstract
The [24]annulene 1 containing four CH2 bridges is surprisingly formed in the twostep organometallic title reaction. The key intermediate is a bismethano-bridged nickelacyclotridecahexaene with two PMe3 ligands. Upon treatment of this complex with PMe3, the final product 1 is formed by reductive elimination.
References
- 1 E. Vogel, H. D. Roth, Angew. Chem. 76 (1964) 145; Angew. Chem. Int. Ed. Engl. 3 (1964) 228.
- 2 G. Wilke, M. Kröner, B. Bogdanović, Angew. Chem. 73 (1961) 755.
- 3 G. Wilke, Pure Appl. Chem. 50 (1978) 677.
- 4 P. Binger, M. J. Doyle, J. McMeeking, C. Krüger, Y.-H. Tsay, J. Organomet. Chem. 135 (1977) 405.
- 5 W. E. Billups, A. J. Blakeney, W. Y. Chow, Org. Synth. 55 (1976) 12.
- 6 3: A solution of 2 (720 mg, 2.26 mmol) in pentane (40 mL), prepared under argon, was treated at −20°C with 0.48 mL (5.52 mmol) of 1 with vigorous stirring. After 3 h at −20°C, the solution was cooled to −78°C for 2 d to complete the crystallization and then filtered by suction through a cold frit. The crystals were dried under high vacuum, and recrystallized from Et2O. Yield: 769 mg (1.97 mmol, 87%), orange-red crystals, m.p. 142°C (decomp.).-1H-NMR (200 MHz, [D8]tetrahydrofuran, TMS): δ = 6.22 (m, H-2, 4J(2,P) = 4.5 Hz); 6.34 (dd, H-3); 6.13 (dd, H-4); 6.00 (d, H-5); 0.91 (dd, H-7, 4J(7,P) = 3.6 Hz, 4J(7,P') = 1.0 Hz); 2.89 (br. d, H-7′, 2J(7,7′ = 8.3 Hz); 1.37 (d, H-8, 2J(8,P)=7.3 Hz).- 13C-NMR (75.5 MHz, [D8]THF, TMS, −30° C): δ = 174.1 (s, C-1, 2J(P,C) + 2J(P,C') = 41.8 Hz); 125.9 (d, C-2, 3J(P,C) + 3J(P,C) = 13.5 Hz); 128.4 (d, C-3); 122.3 (d, C-4); 117.1 (d, C-5); 129.6 (d, C-6); 52.8 (t, C-7); 16.4 (q, C-8, 2J(P,C) + 2J(P',C) = 28.3 Hz).-31P-NMR (32 MHz, [D8]THF): δ = −17.6 (s).-MS: m/z 390 (M+) (for 58Ni).-IR (KBr): v̄ = 1536, 1579, 1609 (m) (C = C) cm−1.
- 7 The results of an elemental analysis were in accord with the given empirical formula.
- 8 C. Krüger, unpublished results.
- 9 4: A solution of 3 (350 mg, 0.89 mmol) in THF (20 mL) was treated at −78°C under argon with 59.8 mL (2.67 mmol) of CO. After several hours' stirring at RT the THF was removed and the residue taken up in pentane. After filtration through a frit, the filtrate was evaporated to dryness and the resulting residue sublimed in a high vacuum (70–90°C) and recrystallized from Et2O. Yield: 130 mg (0.62 mmol, 70%), colorless crystals, m.p. 91°C.-1H-NMR (200 MHz, [D8]THF, TMS): δ = 6.48, 6.34 (m, H-3,6, 3J(3,4) = 9.4 Hz, 3J(5,6) = 9.4 Hz, 4J (3,5) = 0.7 Hz, 4J(4,6) = 0.7 Hz); 6.00, 5.83 (m, H-4,5, 3J (4,5) = 5.9 Hz); 1.62 (d, H-8′, 2J(8,8′) = 3.9 Hz); 0.20 (d, H-8).-13C-NMR (75.5 MHz, CDCl3, TMS): δ = 206.5 (s, C-1); 42.9, 40.0 (s, C-2,7); 124.4, 122.5, 122.1, 120.3 (d, C-3,5,6,4); 21.8 (t, C-8).-MS: m/z 208 (M+).-IR (KBr): v̄ = 1719 (s) (CO) cm−1, 1545, 1627 (m) (CC).-UV (n-hexane): λmax = 195 nm (λ = 13000), 230 sh (5100), 270 (4600).
- 10 5: A solution of 3 (660 mg, 1.68 mmol) in THF (20 mL) was treated under argon with 5 mL (1.7 M, 8.5 mmol) of etheral HCl. After 30 min, and addition of pentane (50 mL), the mixture was extracted with 30 mL of H2O and the organic phase dried over MgSO4. Subsequent filtration over silica gel and evaporation of the filtrate to dryness furnished 292 mg (1.60 mmol, 95%) of a pale-yellow oil, b.p. 30°C/10−3 torr.- 1H-NMR (200 MHz, CDCl3, TMS): δ = 6.5 (m, 3H, H-2,3,4); 6.20 (m, H-5); 5.42 (m, H-6); 2.60 (d, H-7, 3 J(6,7) = 7.2 Hz).- 13C-NMR (75.5 MHz, CDCl3, TMS): δ = 133.3 (s, C-1); 130.7, 130.3, 127.2, 123.4, 122.5 (d, C-2,3,4,5,6); 30.8 (t,C-7).-MS: m/z 182 ( M+).-IR (KBr): v̄ = 1521, 1581 (m) (CC) cm−1.-UV ( n-hexane): λmax = 221 nm ( e = 18000), 265 (3500), 340(11000).
- 11 R. S. Girens, Tetrahedron Lett. 1969, 663.
- 12 5 and 6: A solution of 3 (600 mg, 1.53 mmol) in THF (20 mL) was treated under argon with 1% H2SO4 (10 mL) and, after 30 min, with pentane (50 mL). The organic phase was washed three times with H2O (30 mL) and dried over MgSO4. Flash chromatography on silica gel (15 cm × 3 cm Ø; eluent: pentane) yielded, besides 111 mg (0.61 mmol, 40%) of 5, in the second fraction 153 mg (0.42 mmol, 55%) of red crystals of m.p. 114°C. 6: 1H-NMR (200 MHz, CDCl3, TMS): δ = 2.51 (d, 2H, H-1); 5.35 (br. q, 1H, H-2); 6.17 (m, 1H, H-3); 6.60 (m, 6H, H-4,5,9,10,11,12); 6.37 (br. d, 1H, H-6); 2.75 (s, 2H, H-14).-13C-NMR (75.5 MHz, CDCl3, TMS): δ = 30.6 (t, C-1); 122.3, 123.0, 124.2, 124.5, 127.0 (d, C-2,3,6,9,12); 130.3 (d, C-4); 130.4 (2C), 130.8 (d, C-5, 10,11); 132.5, 132.8, 132.9 (s, C-7,8, 13); 33.2 (t, C-14).-MS: m/z 362 ( M+).- IR (KBr): v̄ = 1487, 1518, 1577 (m) (CC) cm−1.-UV ( n-hexane): λmax = 241 nm (ϵ = 37000), 299 (20800), 340 (13500), 394 (10400).
- 13 7: A solution of 3 (2.0 g, 5.11 mmol) in THF (300 mL) was treated at −78°C under argon with 1 mL (10.22 mmol) of trimethylphosphane. The mixture was allowed to warm to RT over 4d. After removal of the THF the residue was taken up in pentane and the resulting solution filtered through a frit; the filtrate and pentane washings were evaporated to dryness and subjected to work-up by HPLC. Crude yield: 75 mg (0.42 mmol, 8.2%). After HPLC separation: 41 mg (0.11 mmol, 4.5%) red crystals, m.p. 277°C.-1H-NMR (400 MHz, CD2Cl2, TMS): δ = 0.65 (d, H-1, 2 J(1,1′) = 11.8 Hz); 3.18 (d, H-1′); 6.33 (m, H-3), 3J(3,4) = 6.8 Hz); 6.68 (m, H-4).-13C-NMR (75.5 MHz, CD2Cl2, TMS): δ = 31.0 (t, C-1); 126.5 (s, C-2); 124.6 (d, C-3); 128.5 (d, C-4).-MS: m/z 360 ( M+).-IR (KBr): v̄ = 1570 (m) (CC) cm−1.-Uv ( n-hexane): λmax = 263 nm (ϵ = 91100), 338 (22600), 415 (3800).