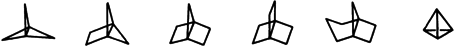The Concept of Strain in Organic Chemistry
Corresponding Author
Prof. Kenneth B. Wiberg
Department of Chemistry, Yale University, New Haven, CT 06511 (USA)
Department of Chemistry, Yale University, New Haven, CT 06511 (USA)Search for more papers by this authorCorresponding Author
Prof. Kenneth B. Wiberg
Department of Chemistry, Yale University, New Haven, CT 06511 (USA)
Department of Chemistry, Yale University, New Haven, CT 06511 (USA)Search for more papers by this authorGraphical Abstract
The ring-strain theory, which Adolf von Baeyer formulated one hundred years ago, has been expanded in many directions; today, strain is discussed in terms of bond-length and bond-angle distortions as well as nonbonding interactions. Only in such terms can the stability of such highly strained compounds as tetra-tert-butyltetrahedrane and [1.1.1]propellane be understood.
Abstract
“Die Ringschließung ist offenbar diejenige Erscheinung, welche am meisten über die räumliche Anordnung der Atome Auskunft geben kann. Wenn eine Kette von 5 und 6 Gliedern sich leicht, eine von weniger oder mehr Gliedern sich schwierig oder auch gar nicht schließen läßt, so müssen dafür offenbar räumliche Gründe vorhanden sein.… Die vier Valenzen des Kohlenstoffatoms wirken in den Richtungen, welche den Mittelpunkt der Kugel mit den Tetraederecken verbinden, und welche miteinander einen Winkel von 109°28′ machen. Die Richtung der Anziehung kann eine Ablenkung erfahren, die jedoch eine mit der Größe der Letzteren wachsende Spannung zur Folge hat,”[
References
- 1 A. von Baeyer, Ber. Dtsch. Chem. Ges. 18 (1885) 2278.
- 2
W. Perkin, Jr.,
Ber. Dtsch. Chem. Ges.
17
(1884)
323.
10.1002/cber.18840170194 Google Scholar
- 3
J. D. Cox,
G. Pilcher:
Thermochemistry of Organic and Organometallic Compounds,
Academic Press, London
1970;
10.1002/9783527619825 Google ScholarJ. B. Pedley, J. Rylance: Sussex-N.P.L. Computer Analyzed Thermochemical Data; Organic and Organometallic Compounds, University of Sussex 1977; D. R. Stull, E. F. Westrum, Jr., C. G. Sinke: The Chemical Thermodynamics of Organic Compounds, Wiley, New York 1969.
- 4 For reviews dealing with strain, see: A. Greenberg, J. L. Liebman: Strained Organic Compounds, Academic Press, New York 1978; M. D. Newton, Mod. Theor. Chem. 4 (1977) 223; A. de Meijere, Angew. Chem. 91 (1979) 867; Angew. Chem. Int. Ed. Engl. 18 (1979) 809.
- 5 K. B. Wiberg, G. B. Ellison, J. J. Wendoloski, J. Am. Chem. Soc. 98 (1976) 1212.
- 6 D. R. Lide, Jr., J. Chem. Phys. 33 (1960) 4363.
- 7 K. B. Wiberg, J. Am. Chem. Soc., in press.
- 8 H. C. Brown, R. B. Johannesen, J. Am. Chem. Soc. 75 (1953) 16.
- 9 H. C. Brown, R. S. Fletcher, J. Am. Chem. Soc. 72 (1950) 1223.
- 10 T. H. Lowry, K. S. Richardson: Mechanism and Theory in Organic Chemistry, 2nd ed., Harper and Row, New York 1981, chap. 8.
- 11 R. W. Taft, Jr. in M. Newman (Ed.): Steric Effects in Organic Chemistry, Wiley, New York 1956.
- 12 F. H. Westheimer, J. E. Mayer, J. Chem. Phys. 14 (1946) 733; F. H. Westheimer in M. Newman (Ed.): Steric Effects in Organic Chemistry, Wiley, New York 1956.
- 13 U. Burkert, N. L. Allinger: Molecular Mechanics (ACS Monograph. 177) Washington, D.C. 1980.
- 14 H. Nakatsaji, J. Am. Chem. Soc. 96 (1974) 24; H. P. Figeys, D. Berckmans, P. Geerlings, J. Mol. Struct. 57 (1979) 271; D. M. Chipman, W. E. Palke, B. J. Kirtman, J. Am. Chem. Soc. 102 (1980) 3377; K. B. Wiberg, J. J. Wendoloski, J. Comput. Chem. 2 (1981) 53.
- 15 R. F. W. Bader, Y. Tal, S. G. Anderson, T. T. Nguyen-Dang, Isr. J. Chem. 19 (1980) 8.
- 16 T. L. Cottrell: The Strengths of Chemical Bonds, 2nd ed., Butterworths, London 1958.
- 17 B. S. Rabinovitch, E. W. Schlag, K. B. Wiberg, J. Chem. Phys. 28 (1958) 504; E. W. Schlag, B. S. Rabinovitch, J. Chem. Phys. 32 (1960) 5996.
- 18 K. B. Wiberg, G. M. Lampman, J. Am. Chem. Soc. 88 (1966) 4429.
- 19 A. Almennigen, O. Bastiansen, P. N. Skancke, Acta Chem. Scand. 15 (1961) 711. For a review of cyclobutane structures, see: F. A. Cotton, B. A. Frenz, Tetrahedron 30 (1974) 1587; T. B. Malloy, Jr., W. J. Lafferty, J. Mol. Spectrosc. 54 (1975) 20; R. M. Moriarty, Top. Stereochem. 8 (1974) 271.
- 20 A. L. Verma, U. F. Murphy, H. J. Bernstein, J. Chem. Phys. 60 (1974) 1540.
- 21 J. L. Franklin, Ind. Eng. Chem. 41 (1949) 1070. Another set of group equivalents for use in calculating strain energies has been proposed by E. M. Engler, J. D. Andose, P. von R. Schleyer, J. Am. Chem. Soc. 95 (1973) 8005.
- 22 K. B. Wiberg, J. Comput. Chem. 5 (1984) 197.
- 23 W. J. Adams, J. J. Geise, L. S. Bartell, J. Am. Chem. Soc. 92 (1970) 5013.
- 24 M. H. Baghal-Vayjouee, S. W. Benson, J. Am. Chem. Soc. 101 (1979) 2838.
- 25 W. Tsang, J. Am. Chem. Soc. 107 (1985) 2872.
- 26 K. B. Wiberg, R. A. Fenoglio, J. Am. Chem. Soc. 90 (1968) 3395.
- 27 The C-H bond in cyclopropane is 0.01 Å shorter than that in propane, and its vibrational frequency is larger than that of the CH2 group of propane: L. N. Ferguson: Highlights of Alicyclic Chemistry, Part I, Franklin Publ. Co., Palisades, NJ, USA 1973, p. 211.
- 28 F. A. L. Anet, Fortschr. Chem. Forsch. 45 (1974) 169.
- 29 M. Saunders, Tetrahedron 23 (1967) 2105.
- 30 K. B. Wiberg, Acc. Chem. Res. 17 (1985) 379.
- 31 P. G. Gassman, M. L. Greenlee, D. A. Dixon, S. Richtsmeier, J. Z. Gougoutas, J. Am. Chem. Soc. 105 (1983) 5865; K. W. Cox, M. D. Harmony, G. Nelson, K. B. Wiberg, J. Chem. Phys. 50 (1969) 1976. (bridgehead C-H bond 11.5° below the plane formed by C-1, C-2, C-4).
- 32 K. B. Wiberg, E. C. Lupton, Jr., D. J. Wasserman, A. de Meijere, S. R. Kass, J. Am. Chem. Soc. 106 (1984) 1740.
- 33 J. V. Paukstelis, J.-L. Kao, J. Am. Chem. Soc. 94 (1972) 4783; P. Gassman, S. M. Bonser, J. Am. Chem. Soc. 105 (1983) 667.
- 34 P. B. Shevlin, A. P. Wolf, J. Am. Chem. Soc. 92 (1970) 406, 5291; H. Ona, H. Yamaguchi, S. Masamune, J. Am. Chem. Soc. 92 (1970) 7495; L. B. Rodewald, H. K. Lee, J. Am. Chem. Soc. 95 (1973) 623, 3084.
- 35 J. E. Douglas, B. S. Rabinovitch, F. S. Looney, J. Chem. Phys. 23 (1955) 315.
- 36 K. B. Wiberg, J. Caringi, unpublished results.
- 37 W. F. Maier, P. von R. Schleyer, J. Am. Chem. Soc. 103 (1981) 1891. This paper gives the strain energies of many alkenes as calculated via molecular mechanics.
- 38 For a review of molecular mechanics as applied to strained alkenes, see O. Ermer: Aspekte von Kraftfeldrechnungen, Wolfgang Baur Verlag, Munich 1981.
- 39 M. Traetteberg, Acta Chem. Scand. B 29 (1975) 29.
- 40 J. Bredt, Justus Liebigs Ann. Chem. 437 (1924) 1.
- 41 J. A. Marshall, Acc. Chem. Res. 13 (1980) 213.
- 42 E. J. Corey, F. A. Carey, R. A. E. Winter, J. Am. Chem. Soc. 87 (1965) 934: R. Bonneau, J. Joussot-Dubien, L. Salem, A. J. Yarwood, J. Am. Chem. Soc. 98 (1976) 4329.
- 43 D. W. Rogers, H. von Voithenberg, N. L. Allinger, J. Org. Chem. 43 (1978) 360.
- 44 A. C. Cope, P. T. Moore, W. R. Moore, J. Am. Chem. Soc. 82 (1960) 1744.
- 45 For reviews an bridgehead double bonds, see: G. Köbrich, Angew. Chem. 85 (1973) 494; Angew. Chem. Int. Ed. Engl. 12 (1973) 464; R. Keese, Angew. Chem. Int. Ed. Engl. 87 (1975) 568 and Angew. Chem. Int. Ed. Engl. 14 (1975) 528; G. L. Buchanan, Chem. Soc. Rev. 3 (1974) 41; see also [37].
- 46 J. R. Wiseman, J. Am. Chem. Soc. 89 (1967) 5966.
- 47 J. A. Marshall, H. Faubl, J. Am. Chem. Soc. 89 (1967) 5965; J. R. Wiseman, H.-F. Chan, C. J. Ahola, J. Am. Chem. Soc. 91 (1969) 2812; J. R. Wiseman, J. A. Chong, J. Am. Chem. Soc. 91 (1969) 7775; J. Am. Chem. Soc. 94 (1972) 8627; H. H. Grootveld, C. Blomberg, F. Bickelhaupt, J. Chem. Soc. Chem. Commun. 1973, 542.
- 48 A. Almenningen, I. M. Anfisen, A. Haaland, Acta Chem. Scand. 24 (1970) 43.
- 49 N. L. Allinger, J. T. Sprague, J. Am. Chem. Soc. 94 (1972) 5734.
- 50 O. Ermer, S. Lifson, Tetrahedron 30 (1974) 2425.
- 51 For recent attempts at preparing tetra-tert-butylethylene, see: A. Krebs, W. Born, B. Kaletta, W. U. Nickel, R. Wolfgang, Tetrahedron Lett. 24 (1983) 4821.
- 52 H.-U. Wagner, G. Szeimies, J. Chandrasekhar, P. von R. Schleyer, J. A. Pople, J. S. Binkley, J. Am. Chem. Soc. 100 (1978) 1210; K. B. Wiberg, G. Bonneville, R. Dempsey, Isr. J. Chem. 23 (1983) 85.
- 53 H.-G. Zoch, G. Szeimies, R. Römer, G. Germain, J.-P. Declercq, Chem. Ber. 116 (1983) 2285; O. Baumgärtel, J. Harnisch, G. Szeimies, M. Van Meerssche, G. Germain, J.-P. Declercq, Chem. Ber. 116 (1983) 2205.
- 54 G. Wipff, K. Morokuma, Tetrahedron Lett. 21 (1980) 4445; K. B. Wiberg, G. Bonneville, R. Dempsey, Isr. J. Chem. 23 (1983) 85.
- 55 N. G. Rondan, M. N. Paddon-Row, P. Caramella, J. Mareda, P. H. Mueller, K. N. Houk, J. Am. Chem. Soc. 104 (1982) 4974.
- 56 R. Huisgen, P. H. J. Ooms, M. Mingen, N. L. Allinger, J. Am. Chem. Soc. 102 (1980) 3951.
- 57 V. Boekelheide, Acc. Chem. Res. 13 (1980) 65.
- 58 K. B. Wiberg, M. G. Matturro, P. J. Okarma, M. E. Jason, J. Am. Chem. Soc. 106 (1984) 2194.
- 59 For related compounds see: N. M. Weinsheker, F. D. Greene, J. Am. Chem. Soc. 90 (1968) 506; R. L. Viavattene, F. D. Greene, L. D. Cheung, R. Majeste, L. M. Trefonas, J. Am. Chem. Soc. 96 (1974) 4342.
- 60 E. Kloster-Jensen, J. Wirtz, Helv. Chim. Acta 58 (1975) 162.
- 61 R. Greenhouse, W. T. Borden, K. Hirotsu, J. Clardy, J. Am. Chem. Soc. 99 (1977) 1664.
- 62 See ref. [4] for many examples of strained compounds containing heteroatoms. For a recent example, see J. G. Radziszewski, J. W. Downing, C. Wentrup, P. Kaszynski, M. Jawdosiuk, P. Kovacic, J. Michl, J. Am. Chem. Soc. 106 (1984) 7996.
- 63 D. A. Dougherty, H. B. Schlegel, K. Mislow, Tetrahedron 34 (1978) 1441; K. B. Wiberg, R. D. Adams, P. J. Okarma, M. G. Matturro, B. Segmuller, J. Am. Chem. Soc. 106 (1984) 2200; see also [37].
- 64 Cyclopropane is commonly taken as having a normal bond angle of 60° and the strain of the ring is added to the final steric energy. Similarly, cyclobutane may be taken as having a normal bond angle of 90°, and the spectroscopically determined C-C-C bending force constant is used. Again, the strain in the ring is added to the final strain energy. Cf. ref. [13] and S. Chang, D. McNally, S. Dhary-Tehrany, M. J. Hickey, R. Boyd, J. Am. Chem. Soc. 92 (1970) 3109.
- 65 L. S. Bartell, J. Am. Chem. Soc. 99 (1977) 3279; N. L. Allinger, D. Hindman, H. Hönig, J. Am. Chem. Soc. 99 (1977) 3282.
- 66 For a review of conformations of carbonyl compounds, see V. Suter, J. Am. Chem. Soc. 101 (1979) 6481.
- 67 S. Kondo, E. Hirota, Y. Morino, J. Mol. Spectrosc. 28 (1968) 471; J. R. Durig, D. A. C. Compton, J. Phys. Chem. 84 (1980) 773.
- 68 K. B. Wiberg, E. Martin, J. Am. Chem. Soc. 107 (1985) 5035.
- 69 A. D. Buckingham, B. D. Utting, Annu. Rev. Phys. Chem. 21 (1970) 287.
- 70 D. E. Williams, J. Chem. Phys. 43 (1965) 4424; D. E. Williams, T. L. Starr, J. Comput. Chem. 1 (1977) 173.
- 71
Electron populations may be calculated by integrating the charge density over regions of space corresponding to the atoms:
R. F. W. Bader,
S. G. Anderson,
A. J. Duke,
J. Am. Chem. Soc.
101
(1979)
1389;
K. B. Wiberg,
J. J. Wendoloski,
Proc. Natl. Acad. Sci. USA
78
(1981)
6561.
Although these populations should not be considered point charges, they give the sense and magnitude of the bond dipole. It may be noted that the C-H bond dipole for most hydrocarbons has the direction
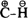
- 72 J. A. Pople, Mod. Theor. Chem. 4 (1977) 1.
- 73 P. Pulay in B. Deb (Ed.): The Force Concept in Chemistry. Van Nostrand, New York 1981.
- 74 T. Hirano, E. Osawa, Croat. Chem. Acta 57 (1984) 1633.
- 75 K. B. Wiberg, S. R. Kass, J. Am. Chem. Soc. 107 (1985) 988.
- 76 K. B. Wiberg, F. H. Walker, J. Am. Chem. Soc. 104 (1982) 5239; K. B. Wiberg, J. Am. Chem. Soc. 105 (1983) 1227; E. Honegger, H. Huber, E. Heilbronner, W. P. Dailey, K. B. Wiberg, J. Am. Chem. Soc. 107 (1985) 7172; K. B. Wiberg, W. P. Dailey, F. H. Walker, S. T. Waddell, L. S. Crocker, M. Newton, J. Am. Chem. Soc. 107 (1985) 7247.
- 77 P. E. Eaton, G. E. Temme III, J. Am. Chem. Soc. 95 (1973) 7508.
- 78 K. B. Wiberg, M. Matturro, Tetrahedron Lett. 22 (1981) 3481.
- 79 M. J. Goldstein, M. S. Benzon, J. Am. Chem. Soc. 94 (1972) 5119.
- 80 L. A. Paquette, J. A. Schwartz, J. Am. Chem. Soc. 92 (1970) 3215; A. Sinnema, F. van Rantwijk, A. J. De Konig, A. M. van Wijk, H. van Bekkem, Tetrahedron 32 (1973) 364; R. Wehrli, D. Bellus, H.-J. Hansen, H. Schmid, Chimia 30 (1979) 416.
- 81 M. Sohn, J. Blum, J. Halpern, J. Am. Chem. Soc. 101 (1979) 2694.
- 82 G. J. Abruscato, T. T. Tidwell, J. Am. Chem. Soc. 92 (1970) 4125; J. Org. Chem. 37 (1972) 4151.
- 83 G. A. Olah, G. K. S. Prakash, J. Org. Chem. 42 (1977) 580.
- 84 G. Maier, S. Pfriem, U. Schäfer, R. Matusch, Angew. Chem. 90 (1978) 552; Angew. Chem. Int. Ed. Engl. 17 (1978) 520.
- 85 C. Y. Lin, A. Krantz, J. Chem. Soc. Chem. Commun. 1972, 111; O. L. Chapman, C. L. McIntosh, J. Pacansky, J. Am. Chem. Soc. 95 (1973) 614.
- 86 K. B. Wiberg, R. R. Squires, J. Am. Chem. Soc. 101 (1979) 5512; J. Am. Chem. Soc. 103 (1981) 4473; K. B. Wiberg, E. J. Martin, R. R. Squires, J. Org. Chem. 50 (1985) 4717.
- 87 K. B. Wiberg, W. Cunningham, unpublished results.