Journal list menu
Export Citations
Download PDFs
Cover Picture
Inside Cover Picture
Inside Cover Picture
- Page: 690
- First Published: 01 March 2024

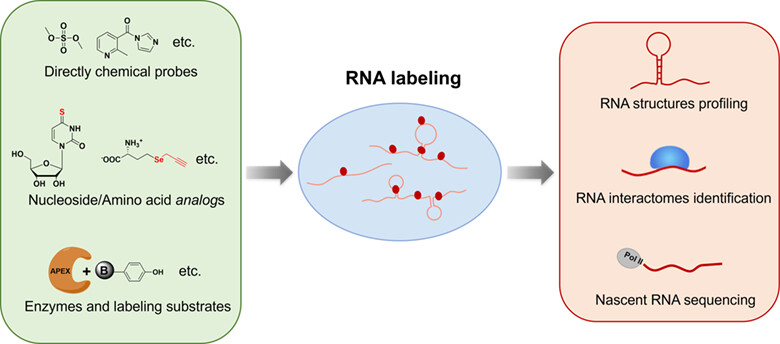
Plenty of advanced methods have been developed for tracing RNA, including directly chemical RNA labeling, metabolic RNA labeling, proximity-dependent RNA labeling, etc. These RNA labeling strategies have greatly enhanced our learning about complex roles and functions of RNA. For the deeper insight of RNA biology, there is still a constant need to develop newer RNA labeling methods. More details are discussed in the article by Weng et al. on page 790—801.
Contents
Concise Reports
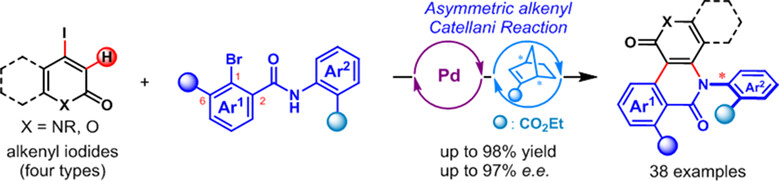
Asymmetric Two-Component Alkenyl Catellani Reaction for the Construction of C—N Axial Chirality
- Pages: 699-704
- First Published: 29 November 2023
Synthesis of Planar-Chiral [2.2]Paracyclophane-Based Oxazole-Pyrimidine Ligands and Application in Nickel-Catalyzed 1,2-Reduction of α,β-Unsaturated Ketones
- Pages: 705-710
- First Published: 27 November 2023
![Synthesis of Planar-Chiral [2.2]Paracyclophane-Based Oxazole-Pyrimidine Ligands and Application in Nickel-Catalyzed 1,2-Reduction of α,β-Unsaturated Ketones](/cms/asset/2f41c81f-0e62-4c5e-ba7b-6866bd4cbac7/cjoc202300575-toc-0001-m.jpg)
The [2.2]paracyclophane-derived oxazole-pyrimidine ligands with planar chirality (PYMCOX) were designed, synthesized and successfully applied in nickel-catalyzed asymmetric 1,2-reduction of α,β-unsaturated ketones, affording the chiral allyilic alcohols with up to 99% yield and 99% ee. Meanwhile, this reduction reaction could be conducted on gram scale without loss of activity and enantioseletivity, and the chiral ligand could be conveniently recovered with high yield.
Enantioselective Synthesis of Benzimidazole Atropisomers Featuring a N-N Axis
- Pages: 711-718
- First Published: 07 December 2023
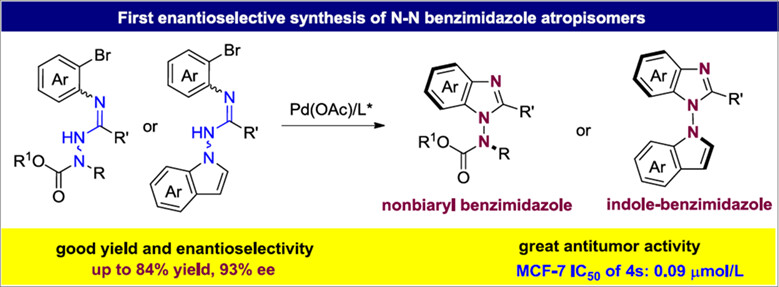
The first enantioselective synthesis of N-N benzimidazole atropisomers via Pd-catalyzed enantioselective intramolecular Buchwald-Hartwig amination reaction was reported. A broad range of nonbiaryl benzimidazole and indole-benzimidazole atropisomers were conveniently accessed in high yields and with excellent enantioselectivities.
Supported Atomically Dispersed Pd Catalyzed Direct Alkoxylation and Allylic Alkylation
- Pages: 719-724
- First Published: 29 November 2023
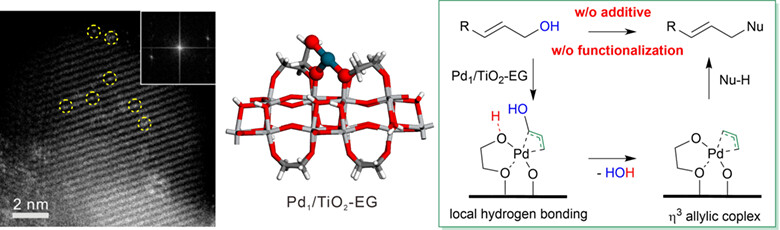
The direct allylic alkylation of nucleophiles using allylic alcohols was realized by supported atomically dispersed Pd1/TiO2-EG under the mild neutral condition without using any additives. The distinct performance was attributed to the hydrogen bonds-assisted removing of –OH at the organic-inorganic interface.
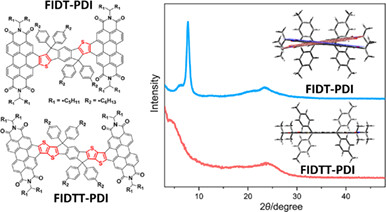
Effect of Hyperconjugation on Photovoltaic Performance in Pseduo-2D Perylene Diimide Derivatives
- Pages: 725-730
- First Published: 06 December 2023
Atroposelective Synthesis of 2-Arylindoles via Chiral Phosphoric Acid-Catalyzed Direct Amination of Indoles
- Pages: 731-735
- First Published: 29 November 2023
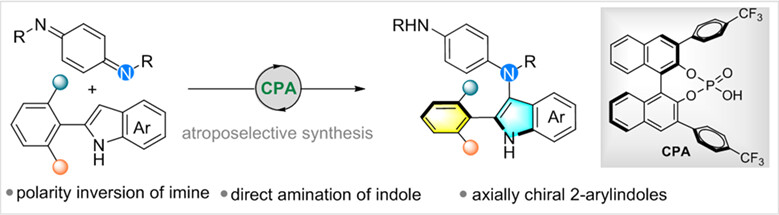
Herein, we developed a general and mild catalytic electrophilic amination of 2-arylindoles with p-quinone diimines to construct axially chiral 2-arylindoles under mild conditions. With the proper choice of a chiral phosphoric acid and substituted 2-arylindoles, the intermolecular C—N bond formation with various p-quinonediimines proceeded with good yields and high stereoselectivities. This strategy provides a powerful method for enantioselective synthesis of 2-arylindole atropisomers which successfully overcame the challenges in catalytic asymmetric construction of axially chiral five-membered heterobiaryls.
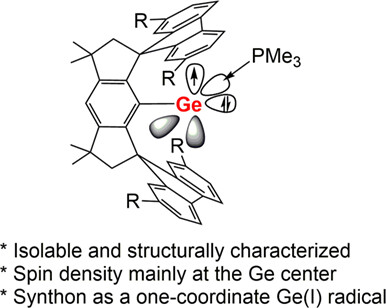
An Isolable Phosphinogermylyne as a Synthon of One-Coordinate GeI Radical
- Pages: 736-742
- First Published: 27 November 2023
Polyaspers A and B, the First Ergosterol-Polyether Adducts with Unprecedented 6/6/6/5/5/6/6/6/6 Nonacyclic Architecture from Aspergillus sp. TJ507
- Pages: 743-751
- First Published: 11 December 2023
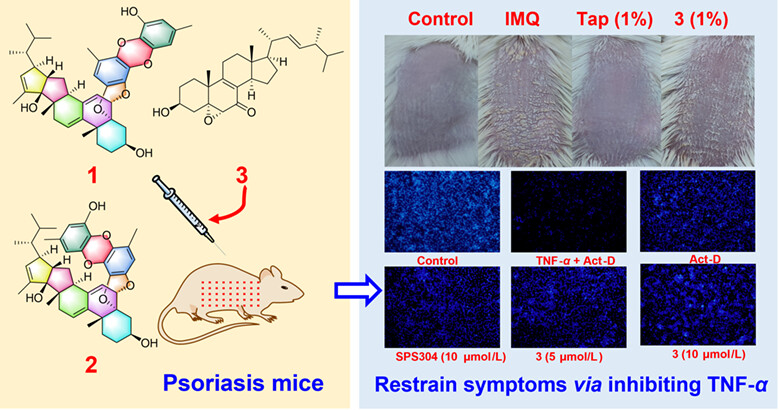
Polyaspers A (1) and B (2), the first ergosterol-polyether adducts, were isolated from Aspergillus sp. TJ507, possessing an unequalled 6/6/6/5/5/6/6/6/6 nonacyclic system. 3 could suppress the inflammatory response simulated with TNF-α in HaCaT cells, and in an imiquimod-induced psoriasis murine model, 3 significantly restrained the development of psoriasis symptoms and reduced the expression of IL-17 and IL-23, presenting an anti-psoriatic effect.
Comprehensive Report
Semicrystalline Unfused Polymer Donors with Backbone Halogenation toward Cost-Effective Organic Solar Cells
- Pages: 752-759
- First Published: 06 December 2023
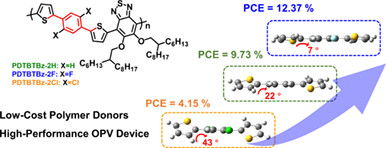
A novel class of low-cost unfused polymer donors was developed with the regulated backbone planarity by halogenation, which facilitates the molecular self-assembly for favorable film morphology. Our results provide insight into the effects of backbone halogenation on the optoelectronic properties of fully unfused polymer donors and demonstrate a simple backbone motif for the further commercialization of cost-effective OSCs.
Recent Advances
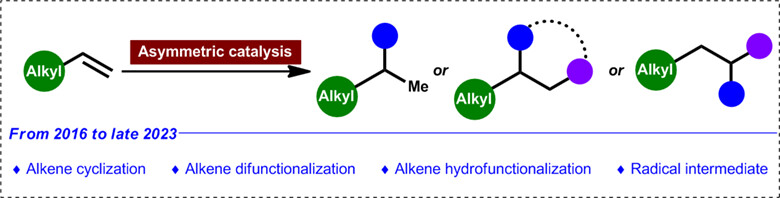
Recent Advances in Enantioselective Reactions of Terminal Unactivated Alkenes
- Pages: 760-776
- First Published: 20 November 2023
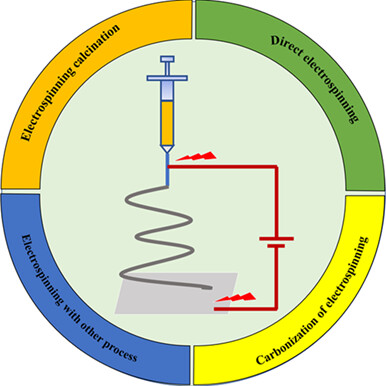
Research on Electromagnetic Wave Absorption Based on Electrospinning Technology†
- Pages: 777-789
- First Published: 20 November 2023
Critical Review
Intracellular RNA Labeling Technologies for the Analysis of RNA Biology
- Pages: 790-801
- First Published: 11 December 2023
Inside Back Cover
Inside Back Cover
- Page: 803
- First Published: 01 March 2024
Psoriasis is a chronic skin disease and the TNF-α is the therapeutic target of this disease. The experiments including SPR and in vivo tests discovered the ergosterols from the fungus Aspergillus sp. TJ507 serve as lead structures for the development of novel TNF-α inhibitory agents in the clinical treatment of psoriasis. More details are discussed in the article by Zhang et al. on page 743—751.
Back Cover
Back Cover
- Page: 804
- First Published: 01 March 2024

Developing novel unfused building blocks with simple synthesis and low cost is essential to advance cost-effective polymer donors in the field of organic solar cells, however, remains challenging. Herein, a class of low-cost and fully unfused polymer donors with precisely regulated backbone planarity via halogenation was designed and synthesized, which possess a four-step synthesis route with over 80% yield from cheap raw chemicals comparable to existing low-cost polymer donors, such as PTQ10. More details are discussed in the article by Li et al. on page 752—759.