An Isolable Phosphinogermylyne as a Synthon of One-Coordinate GeI Radical
Dongmin Wang
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, Guangdong Basic Research Center of Excellence for Functional Molecular Engineering, School of Chemistry, IGCME, Sun Yat-Sen University, Guangzhou, Guangdong, 510275 China
Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorHaonan Chen
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, Guangdong Basic Research Center of Excellence for Functional Molecular Engineering, School of Chemistry, IGCME, Sun Yat-Sen University, Guangzhou, Guangdong, 510275 China
Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorYuhao He
Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorXiaodan Chen
College of Chemistry and Material, Jinan University, Guangzhou, Guangdong, 510632 China
Search for more papers by this authorLi Zhang
School of Electronic Engineering, Guangxi University of Science and Technology, Liuzhou, Guangxi, 545000 China
Search for more papers by this authorCorresponding Author
Gengwen Tan
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, Guangdong Basic Research Center of Excellence for Functional Molecular Engineering, School of Chemistry, IGCME, Sun Yat-Sen University, Guangzhou, Guangdong, 510275 China
Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Hangzhou Normal University, Hangzhou, Zhejiang, 311121 China
E-mail: [email protected]Search for more papers by this authorDongmin Wang
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, Guangdong Basic Research Center of Excellence for Functional Molecular Engineering, School of Chemistry, IGCME, Sun Yat-Sen University, Guangzhou, Guangdong, 510275 China
Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorHaonan Chen
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, Guangdong Basic Research Center of Excellence for Functional Molecular Engineering, School of Chemistry, IGCME, Sun Yat-Sen University, Guangzhou, Guangdong, 510275 China
Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorYuhao He
Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorXiaodan Chen
College of Chemistry and Material, Jinan University, Guangzhou, Guangdong, 510632 China
Search for more papers by this authorLi Zhang
School of Electronic Engineering, Guangxi University of Science and Technology, Liuzhou, Guangxi, 545000 China
Search for more papers by this authorCorresponding Author
Gengwen Tan
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, Guangdong Basic Research Center of Excellence for Functional Molecular Engineering, School of Chemistry, IGCME, Sun Yat-Sen University, Guangzhou, Guangdong, 510275 China
Innovation Center for Chemical Science, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu, 215123 China
Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Hangzhou Normal University, Hangzhou, Zhejiang, 311121 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
Reduction of chlorogermylene MsFluindtBu-GeCl 1 with potassium graphite (KC8) afforded putative germylyne radical MsFluindtBu-Ge 2 as confirmed by electron paramagnetic resonance (EPR) spectroscopy. However, it slowly decayed via C—H bond activation at the fluorenyl moiety to yield a bis(germylene) 3 at room temperature. By using a Lewis base to stabilize the unoccupied p orbital at the GeI radical center, acyclic two-coordinate GeI radicals MsFluindtBu-Ge(IMe4) 4 (IMe4 = 1,3,4,5-tetramethyl-imidazolin-2-ylidene), MsFluindtBu-Ge(IiPr) 5 (IiPr = 1,3-diisopropyl-4,5-dimethyl-imidazolin-2-ylidene), MsFluindtBu-Ge(PMe3) 6 were isolated in crystalline forms. The unpaired electron in 4—6 mainly resides at the Ge 4p orbital as revealed by EPR spectroscopic studies and theoretical calculations. Interestingly, facile ligand exchange of PMe3 in 6 with IMe4 and IiPr was observed to afford 4 and 5, respectively. Moreover, phosphinogermylyne 6 reacted with PhEEPh (E = S, Se), 4-tetrabutylphenylacetylene (Ar'CCH), [CpMo(CO)3]2 and nBu3SnH to furnish germylenes MsFluindtBu-GeEPh (E = S 7, Se 8), MsFluindtBu-GeCH=CHAr’ 9, a germylyne complex MsFluindtBu-Ge≡Mo(CO)2Cp 10 and a Ge(IV) compound MsFluindtBu-GeH2SnnBu3 11, respectively. The reactivity studies demonstrate that 6 can act as a synthon of one-coordinate germylyne radical attributing to labile coordination of trimethylphosphine.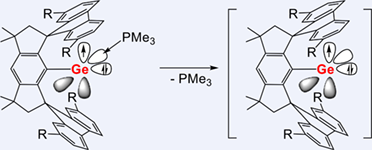
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300581-sup-0001-supinfo.pdfPDF document, 2.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Power, P. P. Persistent and Stable Radicals of the Heavier Main Group Elements and Related Species. Chem. Rev. 2003, 103, 789–809;
(b) Hicks, R. G. In Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds, John Wiley & Sons, Ltd., 2010;
10.1002/9780470666975 Google Scholar(c) Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–7088; (d) Chivers, T.; Konu, J. In Comprehensive Inorganic Chemistry II, 2nd ed., Ed.: K. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 349–373;10.1016/B978-0-08-097774-4.00116-9 Google Scholar(e) Martin, C. D.; Soleilhavoup, M.; Bertrand, G. Carbene-stabilized main group radicals and radical ions. Chem. Sci. 2013, 4, 3020–3030; (f) Chandra Mondal, K.; Roy, S.; Roesky, H. W. Silicon based radicals, radical ions, diradicals and diradicaloids. Chem. Soc. Rev. 2016, 45, 1080–1111; (g) Su, Y.; Kinjo, R. Boron-containing radical species. Coord. Chem. Rev. 2017, 352, 346–378; (h) Tan, G.; Wang, X. Isolable Radical Ions of Main-Group Elements: Structures, Bonding and Properties. Chin. J. Chem. 2018, 36, 573–586; (i) Feng, Z.; Tang, S.; Su, Y.; Wang, X. Recent advances in stable main group element radicals: preparation and characterization. Chem. Soc. Rev. 2022, 51, 5930–5973.
- 2 Gomberg, M. An instance of trivalent carbon: triphenylmethyl. J. Am. Chem. Soc. 1900, 22, 757–771.
- 3(a) Olmstead, M. M.; Pu, L.; Simons, R. S.; Power, P. P. Reduction of Ge(Cl)C6H3Mes2-2,6 to give the cyclotrigermenyl radical (GeC6H3Mes2-2,6)3· and the trigermenyl anion salt K(GeC6H3Mes2-2,6)3. Chem. Commun. 1997, 1595–1596;
(b) Sekiguchi, A.; Fukawa, T.; Nakamoto, M.; Lee, V. Y.; Ichinohe, M. Isolable Silyl and Germyl Radicals Lacking Conjugation with π-Bonds: Synthesis, Characterization, and Reactivity. J. Am. Chem. Soc. 2002, 124, 9865–9869;
(c) Cox, H.; Hitchcock, P. B.; Lappert, M. F.; Pierssens, L. J. M. A 1,3-Diaza- 2,4-distannacyclobutanediide: Synthesis, Structure, and Bonding. Angew. Chem. Int. Ed. 2004, 43, 4500–4504;
(d) Cui, C.; Brynda, M.; Olmstead, M. M.; Power, P. P. Synthesis and Characterization of the Non-Kekulé, Singlet Biradicaloid Ar‘Ge(μ-NSiMe3)2GeAr‘ (Ar‘ = 2,6-Dipp2C6H3, Dipp = 2,6-i-Pr2C6H3). J. Am. Chem. Soc. 2004, 126, 6510–6511;
(e) Ishida, Y.; Sekiguchi, A.; Kobayashi, K.; Nagase, S. 1,6,7-Trigermabicyclo[4.1.0]hept-3-en-7-yl: The Isolable Bicyclic Germyl Radical. Organometallics 2004, 23, 4891–4896;
(f) Förster, C.; Klinkhammer, K. W.; Tumanskii, B.; Krüger, H.-J.; Kelm, H. Stable Mononuclear Lead(III) Compound: A Lead-Centered Radical. Angew. Chem. Int. Ed. 2007, 46, 1156–1159;
(g) Drost, C.; Griebel, J.; Kirmse, R.; Lönnecke, P.; Reinhold, J. A Stable and Crystalline Triarylgermyl Radical: Structure and EPR Spectra. Angew. Chem. Int. Ed. 2009, 48, 1962–1965;
(h) Wang, X.; Peng, Y.; Olmstead, M. M.; Fettinger, J. C.; Power, P. P. An Unsymmetric Oxo/Imido-Bridged Germanium-Centered Singlet Diradicaloid. J. Am. Chem. Soc. 2009, 131, 14164–14165;
(i) Lee, V. Y.; Sekiguchi, A., Organometallic Compounds of Low- Coordinate Si, Ge, Sn and Pb: From Phantom Species to Stable Compounds. In Organometallic Compounds of Low-Coordinate Si, Ge, Sn and Pb: From Phantom Species to Stable Compounds, Wiley, 2010;
10.1002/9780470669266 Google Scholar(j) Chia, S.-P.; Carter, E.; Xi, H.-W.; Li, Y.; So, C.-W. Group II Metal Complexes of the Germylidendiide Dianion Radical and Germylidenide Anion. Angew. Chem. Int. Ed. 2014, 53, 8455–8458; (k) Sugahara, T.; Guo, J.-D.; Hashizume, D.; Sasamori, T.; Tokitoh, N. Reversible Isomerizations between 1,4-Digermabenzenes and 1,4-Digerma-Dewar-benzenes: Air-Stable Activators for Small Molecules. J. Am. Chem. Soc. 2019, 141, 2263–2267; (l) Reinhold, C. R. W.; Schmidtmann, M.; Tumanskii, B.; Müller, T. Radicals and Anions of Siloles and Germoles. Chem. Eur. J. 2021, 27, 12063–12068.
- 4(a) Hudson, A.; Lappert, M. F.; Lednor, P. W. Subvalent Group 4B metal alkyls and amides. Part 4. An electron spin resonance study of some long-lived photochemically synthesised trisubstituted silyl, germyl, and stannyl radicals. J. Chem. Soc., Dalton Trans. 1976, 2369–2375;
10.1039/dt9760002369 Google Scholar(b) Hildenbrand, D. L.; Lau, K. H.; Sanjurjo, A. Experimental Thermochemistry of the SiCl and SiBr Radicals; Enthalpies of Formation of Species in the Si−Cl and Si−Br Systems. J. Phys. Chem. A 2003, 107, 5448–5451; (c) Lai, T. Y.; Tao, L.; Britt, R. D.; Power, P. P. Reversible Sn–Sn Triple Bond Dissociation in a Distannyne: Support for Charge-Shift Bonding Character. J. Am. Chem. Soc. 2019, 141, 12527–12530; (d) Tao, L.; Lai, T. Y.; Power, P. P.; Britt, R. D. Germanium Hydride Radical Trapped during the Photolysis/Thermolysis of Diarylgermylene. Inorg. Chem. 2019, 58, 15034–15038.
- 5(a) Stender, M.; Phillips, A. D.; Wright, R. J.; Power, P. P. Synthesis and Characterization of a Digermanium Analogue of an Alkyne. Angew. Chem. Int. Ed. 2002, 41, 1785–1787;
10.1002/1521-3773(20020517)41:10<1785::AID-ANIE1785>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar(b) Sekiguchi, A.; Kinjo, R.; Ichinohe, M. A Stable Compound Containing a Silicon-Silicon Triple Bond. Science 2004, 305, 1755–1757; (c) Sasamori, T.; Hironaka, K.; Sugiyama, Y.; Takagi, N.; Nagase, S.; Hosoi, Y.; Furukawa, Y.; Tokitoh, N. Synthesis and Reactions of a Stable 1,2-Diaryl-1,2-dibromodisilene: A Precursor for Substituted Disilenes and a 1,2-Diaryldisilyne. J. Am. Chem. Soc. 2008, 130, 13856–13857; (d) Li, J.; Schenk, C.; Goedecke, C.; Frenking, G.; Jones, C. A Digermyne with a Ge–Ge Single Bond That Activates Dihydrogen in the Solid State. J. Am. Chem. Soc. 2011, 133, 18622–18625; (e) Suzuki, K.; Matsuo, T.; Hashizume, D.; Fueno, H.; Tanaka, K.; Tamao, K. A Planar Rhombic Charge-Separated Tetrasilacyclobutadiene. Science 2011, 331, 1306–1309; (f) Suzuki, K.; Numata, Y.; Fujita, N.; Hayakawa, N.; Tanikawa, T.; Hashizume, D.; Tamao, K.; Fueno, H.; Tanaka, K.; Matsuo, T. A stable free tetragermacyclobutadiene incorporating fused-ring bulky EMind groups. Chem. Commun. 2018, 54, 2200–2203.
- 6(a) Wang, D.; Chen, W.; Zhai, C.; Zhao, L.; Ye, S.; Tan, G. Monosubstituted Doublet Sn(I) Radical Featuring Substantial Unquenched Orbital Angular Momentum. J. Am. Chem. Soc. 2023, 145, 6914–6920; (b) Wang, D.; Zhai, C.; Chen, Y.; He, Y.; Chen, X.-d.; Wang, S.; Zhao, L.; Frenking, G.; Wang, X.; Tan, G. An isolable germylyne radical with a one-coordinate germanium atom. Nat. Chem. 2023, 15, 200–205.
- 7(a) Wu, M.; Chen, W.; Wang, D.; Chen, Y.; Ye, S.; Tan, G. Triplet Bismuthinidenes Featuring Unprecedented Giant and Positive Zero Field Splittings. Natl. Sci. Rev. 2023, 10, nwad169; (b) Wu, M.; Li, H.; Chen, W.; Wang, D.; He, Y.; Xu, L.; Ye, S.; Tan, G. A triplet stibinidene. Chem 2023, 9, 2573–2584; (c) The same bismuthinidene has been reported during the same period: Pang, Y.; Nöthling, N.; Leutzsch, M.; Kang, L.; Bill, E.; van Gastel, M.; Reijerse, E.; Goddard, R.; Wagner, L.; SantaLucia, D.; DeBeer, S.; Neese, F.; Cornella, J. Synthesis and isolation of a triplet bismuthinidene with a quenched magnetic response. Science 2023, 380, 1043–1048.
- 8 Woodul, W. D.; Carter, E.; Müller, R.; Richards, A. F.; Stasch, A.; Kaupp, M.; Murphy, D. M.; Driess, M.; Jones, C. A Neutral, Monomeric Germanium(I) Radical. J. Am. Chem. Soc. 2011, 133, 10074–10077.
- 9(a) Lu, X.; Cheng, H.; Meng, Y.; Wang, X.; Hou, L.; Wang, Z.; Chen, S.; Wang, Y.; Tan, G.; Li, A.; Wang, W. A Two-Coordinate Neutral Germylene Supported by a β-Diketiminate Ligand in the Radical State. Organometallics 2017, 36, 2706–2709; (b) Dai, Y.; Bao, M.; Wang, W.; Xie, Z.; Liu, C.; Su, Y. Crystalline Germanium-Dipyrromethene Radicals: from a Delocalized Neutral to a Localized Cation. Chin. J. Chem. 2022, 40, 2387–2392; (c) Kodama, T.; Uchida, K.; Nakasuji, C.; Kishi, R.; Kitagawa, Y.; Tobisu, M. Open-Shell Germylene Stabilized by a Phenalenyl-Based Ligand. Inorg. Chem. 2023, 62, 7861–7867.
- 10 Siddiqui, M. M.; Sarkar, S. K.; Sinhababu, S.; Ruth, P. N.; Herbst-Irmer, R.; Stalke, D.; Ghosh, M.; Fu, M.; Zhao, L.; Casanova, D.; Frenking, G.; Schwederski, B.; Kaim, W.; Roesky, H. W. Isolation of Transient Acyclic Germanium(I) Radicals Stabilized by Cyclic Alkyl(amino) Carbenes. J. Am. Chem. Soc. 2019, 141, 1908–1912.
- 11 Li, Y.; Chan, Y.-C.; Leong, B.-X.; Li, Y.; Richards, E.; Purushothaman, I.; De, S.; Parameswaran, P.; So, C.-W. Trapping a Silicon(I) Radical with Carbenes: A Cationic cAAC–Silicon(I) Radical and an NHC–Parent- Silyliumylidene Cation. Angew. Chem. Int. Ed. 2017, 56, 7573–7578.
- 12(a) Inoue, S.; Ichinohe, M.; Sekiguchi, A. Isolable Silylene Anion Radical: Structural Characteristics in the Solid State and in Solution. J. Am. Chem. Soc. 2007, 129, 6096–6097; (b) Inoue, S.; Ichinohe, M.; Sekiguchi, A. Isolable Alkali-Metal-Substituted Silyl Radicals (tBu2MeSi)2SiM (M = Li, Na, K): Electronically and Sterically Accessible Planar Silyl Radicals. Organometallics 2008, 27, 1358–1360; (c) Holzner, R.; Reiter, D.; Frisch, P.; Inoue, S. DMAP-stabilized bis(silyl)silylenes as versatile synthons for organosilicon compounds. RSC Adv. 2020, 10, 3402–3406; (d) Lim, L. F.; Judd, M.; Vasko, P.; Gardiner, M. G.; Pantazis, D. A.; Cox, N.; Hicks, J. Crystalline Germanium(I) and Tin(I) Centered Radical Anions. Angew. Chem. Int. Ed. 2022, 61, e202201248.
- 13(a) Lee, V. Y.; Sekiguchi, A. Si-, Ge-, and Sn-Centered Free Radicals: From Phantom Species to Grams-Order-Scale Materials. Eur. J. Inorg. Chem. 2005, 2005, 1209–1222; (b) Lee, V. Y.; Sekiguchi, A. Stable Silyl, Germyl, and Stannyl Cations, Radicals, and Anions: Heavy Versions of Carbocations, Carbon Radicals, and Carbanions. Acc. Chem. Res. 2007, 40, 410–419.
- 14(a) Sharma, M. K.; Ebeler, F.; Glodde, T.; Neumann, B.; Stammler, H.-G.; Ghadwal, R. S. Isolation of a Ge(I) Diradicaloid and Dihydrogen Splitting. J. Am. Chem. Soc. 2020, 143, 121–125; (b) Sharma, M. K.; Rottschäfer, D.; Glodde, T.; Neumann, B.; Stammler, H.-G.; Ghadwal, R. S. An Open-Shell Singlet SnI Diradical and H2 Splitting. Angew. Chem. Int. Ed. 2021, 60, 6414–6418.
- 15 He, Y.; Dai, C.; Wang, D.; Zhu, J.; Tan, G. Phosphine-Stabilized Germylidenylpnictinidenes as Synthetic Equivalents of Heavier Nitrile and Isocyanide in Cycloaddition Reactions with Alkynes. J. Am. Chem. Soc. 2022, 144, 5126–5135.
- 16(a) Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8;
10.1107/S2053273314026370 Google Scholar(b) X-ray data are available free of charge from the Cambridge crystallographic Data Centre under reference numbers CCDC 2203964 (3), 2203965 (4), 2203966 (5), 2203967 (6), 2203968 (7), 2203969 (8), 2203970 (9), 2203971 (10), 2203972 (11).
- 17 Hadlington, T. J.; Hermann, M.; Li, J.; Frenking, G.; Jones, C. Activation of H2 by a Multiply Bonded Amido–Digermyne: Evidence for the Formation of a Hydrido–Germylene. Angew. Chem. Int. Ed. 2013, 52, 10199–10203.
- 18 Krebs, K. M.; Hanselmann, D.; Schubert, H.; Wurst, K.; Scheele, M.; Wesemann, L. Phosphine-Stabilized Digermavinylidene. J. Am. Chem. Soc. 2019, 141, 3424–3429.
- 19 Johnson, E. R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A. J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506.
- 20 Pranckevicius, C.; Weber, M.; Krummenacher, I.; Phukan, A. K.; Braunschweig, H. Phosphinoborylenes as stable sources of fleeting borylenes. Chem. Sci. 2020, 11, 11055–11059.
- 21(a) Sasamori, T.; Sugahara, T.; Agou, T.; Guo, J.-D.; Nagase, S.; Streubel, R.; Tokitoh, N. Synthesis and Characterization of a 1,2-Digermabenzene. Organometallics 2015, 34, 2106–2109; (b) Sugahara, T.; Guo, J.-D.; Sasamori, T.; Nagase, S.; Tokitoh, N. Regioselective Cyclotrimerization of Terminal Alkynes Using a Digermyne. Angew. Chem. Int. Ed. 2018, 57, 3499–3503.
- 22(a) Simons, R. S.; Power, P. P. (η5-C5H5)(CO)2MoGeC6H3-2,6-Mes2: A Transition-Metal Germylyne Complex. J. Am . Chem. Soc. 1996, 118, 11966–11967; (b) Pu, L.; Twamley, B.; Haubrich, S. T.; Olmstead, M. M.; Mork, B. V.; Simons, R. S.; Power, P. P. Triple Bonding to Germanium: Characterization of the Transition Metal Germylynes (η5-C5H5)(CO)2M⋮Ge-C6H3-2,6-Mes2 (M = Mo, W; Mes = −C6H2-2,4,6-Me3) and (η5-C5H5)(CO)2M⋮Ge-C6H3-2,6-Trip2 (M = Cr, Mo, W; Trip = −C6H2-2,4,6-i-Pr3) and the Related Single Bonded Metallogermylenes (η5-C5H5)(CO)3M-G̈e-C6H3-2,6-Trip2 (M = Cr, W). J. Am. Chem. Soc. 2000, 122, 650–656; (c) Queen, J. D.; Phung, A. C.; Caputo, C. A.; Fettinger, J. C.; Power, P. P. Metathetical Exchange between Metal–Metal Triple Bonds. J. Am. Chem. Soc. 2020, 142, 2233–2237.




