Recent Advances in Enantioselective Reactions of Terminal Unactivated Alkenes
Qihang Guo
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
ZJU-Hangzhou Global Scientific and Technological Innovation Center, Hangzhou, Zhejiang, 311215 China
‡These authors contributed equally to this work.
Search for more papers by this authorXuzhong Shen
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
‡These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Zhan Lu
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
E-mail: [email protected]Search for more papers by this authorQihang Guo
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
ZJU-Hangzhou Global Scientific and Technological Innovation Center, Hangzhou, Zhejiang, 311215 China
‡These authors contributed equally to this work.
Search for more papers by this authorXuzhong Shen
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
‡These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Zhan Lu
Center of Chemistry for Frontier Technologies, Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310058 China
College of Chemistry, Zhengzhou University, Zhengzhou, Henan, 450001 China
E-mail: [email protected]Search for more papers by this authorAbstract
Comprehensive Summary
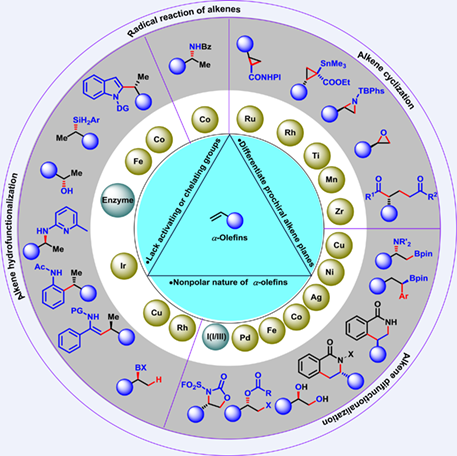
α-Olefins as aliphatic terminal alkenes could be obtained easily from numerous contemporary synthetic reactions as well as petrochemical industry, and also found in natural products. Compared to the alkenes attaching the directing groups or activating group, the catalytic asymmetric reaction of unactivated terminal alkenes presents great challenges due to the weak electron effect and small steric hindrance effect. This review mainly summarizes the latest progress of the asymmetric reaction of unactivated terminal olefins since 2016.
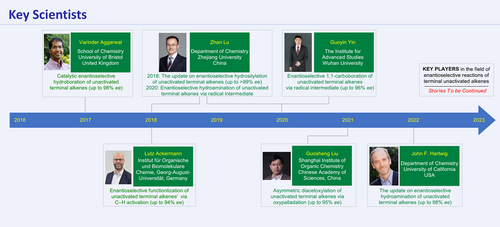
Key Scientists
References
- 1(a) Ananikov, V. P.; Tanaka, M. Hydrofunctionalization, Springer, Heidelberg, 2013;
10.1007/978-3-642-33735-2 Google Scholar(b) Torres Galvis, H. M.; de Jong, K. P. Catalysts for Production of Lower Olefins from Synthesis Gas: A Review. ACS Catal. 2013, 3, 2130−2149.
- 2(a) Chen, J.; Lu, Z. Asymmetric Hydrofunctionalization of Minimally Functionalized Alkenes via Earth Abundant Transition Metal Catalysis. Org. Chem. Front. 2018, 5, 260–272;
(b) Kennemur, J. L.; Maji, R.; Scharf, M. J.; List, B. Catalytic Asymmetric Hydroalkoxylation of C−C Multiple Bonds. Chem. Rev. 2021, 121, 14649–14681;
(c) Sun, X.-Y.; Yao, B.-Y.; Xuan, B.; Xiao, L.-J.; Zhou, Q.-L. Recent Advances in Nickel-catalyzed Asymmetric Hydrofunctionalization of Alkenes. Chem. Catal. 2022, 2, 3140–3162.
10.1016/j.checat.2022.10.020 Google Scholar
- 3(a) Sorádová, Z.; Šebesta, R. Enantioselective Cu-Catalyzed Functionalizations of Unactivated Alkenes. ChemCatChem 2016, 8, 2581–2588; (b) Wang, Z.-X.; Bai, X.-Y.; Li, B.-J. Metal-Catalyzed Substrate-Directed Enantioselective Functionalization of Unactivated Alkenes. Chin. J. Chem. 2019, 37, 1174–1180; (c) Wang, X.-X.; Lu, X.; Li, Y.; Wang, J.-W.; Fu, Y. Recent Advances in Nickel-catalyzed Reductive Hydroalkylation and Hydroarylation of Electronically Unbiased Alkenes. Sci. China Chem. 2020, 63, 1586–1600; (d) Dong, B.; Shen, J.; Xie, L.-G. Recent Developments on 1,2-Difunctionalization and Hydrofunctionalization of Unactivated Alkenes and Alkynes Involving C–S bond Formation. Org. Chem. Front. 2023, 10, 1322–1345.
- 4(a) Oishi, T.; Hirama, M. Highly Enantioselective Dihydroxylation of trans-Disubstituted and Monosubstituted Olefins. J. Org. Chem. 1989, 54, 5834–5835; (b) Jacobsen, E. N.; Markó, I.; Mungall, W. S.; Schröder, G.; Sharpless, K. B. Asymmetric Dihydroxylation via Ligand-accelerated Catalysis. J. Am. Chem. Soc. 1988, 110, 1968–1970; (c) Uozumi, Y.; Hayashi, T. Catalytic Asymmetric Synthesis of Optically Active 2-Alkanols via Hydrosilylation of 1-Alkenes with A Chiral Monophosphine-palladium Catalyst. J. Am. Chem. Soc. 1991, 113, 9887–9888; (d) Kondakov, D. Y.; Negishi, E. Zirconium-Catalyzed Enantioselective Alkylalumination of Monosubstituted Alkenes Proceeding via Noncyclic Mechanism. J. Am. Chem. Soc. 1996, 118, 1577–1578; (e) Davies, H. M. L.; Bruzinski, P. R.; Lake, D. H.; Kong, N.; Fall, M. J. Asymmetric Cyclopropanations by Rhodium(II) N-(Arylsulfonyl)prolinate Catalyzed Decomposition of Vinyldiazomethanes in the Presence of Alkenes. Practical Enantioselective Synthesis of the Four Stereoisomers of 2-Phenylcyclopropan-1-amino Acid. J. Am. Chem. Soc. 1996, 118, 6897–6907; (f) Suematsu, H.; Kanchiku, S.; Uchida, T.; Katsuki, T. Construction of Aryliridium−Salen Complexes: Enantio- and Cis-Selective Cyclopropanation of Conjugated and Nonconjugated Olefins. J. Am. Chem. Soc. 2008, 130, 10327–10337; (g) Kliman, L. T.; Mlynarski, S. N.; Morken, J. P. Aziridination of Alkenes with Trichloroethoxysulfonyl Azide (TcesN3). Chem. Commun. 2009, 4266–4268; (h) Kim, C.; Uchida, T.; Katsuki, T. Asymmetric Olefin Aziridination Using A Newly Designed Ru(CO)(salen) Complex as the Catalyst. Chem. Commun. 2012, 48, 7188–7190; (i) Berkessel, A.; Günther, T.; Wang, Q.; Neudörfl, J.-M. Titanium Salalen Catalysts Based on cis-1,2-Diaminocyclohexane: Enantioselective Epoxidation of Terminal Non-Conjugated Olefins with H2O2. Angew. Chem. Int. Ed. 2013, 52, 8467–8471; (j) Song, G.; Wylie, W. N. O.; Hou, Z. Enantioselective C–H Bond Addition of Pyridines to Alkenes Catalyzed by Chiral Half-Sandwich Rare-Earth Complexes. J. Am. Chem. Soc. 2014, 136, 12209–12212.
- 5 Coombs, J. R.; Morken, J. P. Catalytic Enantioselective Functionalization of Unactivated Terminal Alkenes. Angew. Chem. Int. Ed. 2016, 55, 2636–2649.
- 6 Wang, Z.; Yin, H.; Fu, G. C. Catalytic Enantioconvergent Coupling of Secondary and Tertiary Electrophiles with Olefins. Nature 2018, 563, 379–383.
- 7(a) de Meijere, A.; Kozhushkov, S. I.; Schill, H. Three-Membered- Ring-Based Molecular Architectures. Chem. Rev. 2006, 106, 4926–4996; (b) Chen, D. Y. K.; Pouwer, R. H.; Richard, J. A. Recent Advances in the Total Synthesis of Cyclopropane-containing Natural Products. Chem. Soc. Rev. 2012, 41, 4631–4642; (c) Ebner, C.; Carreira, E. M. Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev. 2017, 117, 11651–11679; (d) Talele, T. T. The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem. 2016, 59, 8712–8756.
- 8(a) Zhu, S.; Xu, X.; Perman, J. A.; Zhang, P. A General and Efficient Cobalt(II)-Based Catalytic System for Highly Stereoselective Cyclopropanation of Alkenes with α-Cyanodiazoacetates. J. Am. Chem. Soc. 2010, 132, 12796–12799; (b) Lo, M. M.-C.; Fu, G. C. A New Class of Planar−Chiral Ligands: Synthesis of a C2-Symmetric Bisazaferrocene and Its Application in the Enantioselective Cu(I)-Catalyzed Cyclopropanation of Olefins. J. Am. Chem. Soc. 1998, 120, 10270–10271; (c) Davies, H. M. L.; Bruzinski, P. R.; Lake, D. H.; Kong, N.; Fall, M. J. Asymmetric Cyclopropanations by Rhodium(II) N-(Arylsulfonyl)prolinate Catalyzed Decomposition of Vinyldiazomethanes in the Presence of Alkenes. Practical Enantioselective Synthesis of the Four Stereoisomers of 2-Phenylcyclopropan-1-amino Acid. J. Am. Chem. Soc. 1996, 118, 6897–6907; (d) Davies, H. M. L.; Bruzinski, P. R.; Lake, D. H.; Kong, N.; Fall, M. J. Asymmetric Cyclopropanations by Rhodium(II) N-(Arylsulfonyl)prolinate Catalyzed Decomposition of Vinyldiazomethanes in the Presence of Alkenes. Practical Enantioselective Synthesis of the Four Stereoisomers of 2-Phenylcyclopropan-1-amino Acid. J. Am. Chem. Soc. 1996, 118, 6897–6907; (e) Xu, X.; Wang, Y.; Cui, X.; Wojtas, L.; Zhang, X. P. Metalloradical Activation of α-Formyldiazoacetates for the Catalytic Asymmetric Radical Cyclopropanation of Alkenes. Chem. Sci. 2017, 8, 4347–4351; (f) Lindsay, V. N. G.; Nicolas, C.; Charette, A. B. Asymmetric Rh(II)-Catalyzed Cyclopropanation of Alkenes with Diacceptor Diazo Compounds: p-Methoxyphenyl Ketone as a General Stereoselectivity Controlling Group. J. Am. Chem. Soc. 2011, 133, 8972–8981.
- 9(a) RulliHre, P.; Benoit, G.; Allouche, E. M. D., Charette, A. B. Safe Facile Access to Nonstabilized Diazoalkanes Using Continuous Flow Technology. Angew. Chem. Int. Ed. 2018, 57, 5777–5782; (b) Greb, A.; Poh, J.-S.; Greed, S.; Battilocchio, C.; Pasau, P.; Blakemore, D. C.; Ley, S. V. A Versatile Route to Unstable Diazo Compounds via Oxadiazolines and their Use in Aryl–Alkyl Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2017, 56, 16602–16605; (c) Schreiner, P. R.; Reisenauer, H. P.; Ley, D.; Gerbig, D.; Wu, C.-H.; Allen, W. D. Methylhydroxycarbene: Tunneling Control of a Chemical Reaction. Science 2011, 332, 1300–1303; (d) Eckhardt, A. K.; Schreiner, P. R. Spectroscopic Evidence for Aminomethylene (H−C̈−NH2)—The Simplest Amino Carbene. Angew. Chem. Int. Ed. 2018, 57, 5248–5252; (e) Benoit, G.; Charette, A. B. Diastereoselective Borocyclopropanation of Allylic Ethers Using a Boromethylzinc Carbenoid. J. Am. Chem. Soc. 2017, 139, 1364–1367; (f) Herlé, B.; Holstein, P. M.; Echavarren, A. M. Stereoselective cis-Vinylcyclopropanation via a Gold(I)-Catalyzed Retro-Buchner Reaction under Mild Conditions. ACS Catal. 2017, 7, 3668–3675.
- 10 Montesinos-Magraner, M.; Costantini, M.; Ramírez-Contreras, R.; Muratore, M. E.; Johansson, M. J.; Mendoza, A. General Cyclopropane Assembly by Enantioselective Transfer of a Redox-Active Carbene to Aliphatic Olefins. Angew. Chem. Int. Ed. 2019, 58, 5930–5935.
- 11 Caló, F. P.; Zimmer, A.; Bistoni, G.; Fürstner, A. From Serendipity to Rational Design: Heteroleptic Dirhodium Amidate Complexes for Diastereodivergent Asymmetric Cyclopropanation. J. Am. Chem. Soc. 2022, 144, 7465−7478.
- 12(a) Sweeney, J. B. Aziridines: Epoxides’ Ugly Cousins? Chem. Soc. Rev. 2002, 31, 247−258; (b) Aziridines and Epoxides in Organic Synthesis, Ed.: Yudin, A. K., Wiley-VCH, Weinheim, 2006; (c) Singh, G. S.; D’hooghe, M.; De Kimpe, N. Synthesis and Reactivity of C–Heteroatom-Substituted Aziridines. Chem. Rev. 2007, 107, 2080−2135.
- 13(a) Ruppel, J. V.; Jones, J. E.; Huff, C. A.; Kamble, R. M.; Chen, Y.; Zhang, X. P. A Highly Effective Cobalt Catalyst for Olefin Aziridination with Azides: Hydrogen Bonding Guided Catalyst Design. Org. Lett. 2008, 10, 1995–1998; (b) Suarez, A. I. O.; Jiang, H.; Zhang, X. P.; de Bruin, B. The Radical Mechanism of Cobalt(ii) Porphyrin-catalyzed Olefin Aziridination and the Importance of Cooperative H-bonding. Dalton Trans. 2011, 40, 5697–5705.
- 14 Boquet, V.; Nasrallah, A.; Dana, A. L.; Brunard, E.; Di Chenna, P. H.; Duran, F. J.; Retailleau, P.; Darses, B.; Sircoglou, M.; Dauban, P. Rhodium(II)-Catalyzed Enantioselective Intermolecular Aziridination of Alkenes. J. Am. Chem. Soc. 2022, 144, 17156−17164.
- 15(a) Xia, Q.-H.; Ge, H.-Q.; Ye, C.-P.; Liu, Z.-M.; Su, K.-X. Advances in Homogeneous and Heterogeneous Catalytic Asymmetric Epoxidation. Chem. Rev. 2005, 105, 1603–1662; (b) De Faveri, G.; Ilyashenko, G.; Watkinson, M. Recent Advances in Catalytic Asymmetric Epoxidation Using the Environmentally Benign Oxidant Hydrogen Peroxide and Its Derivatives. Chem. Soc. Rev. 2011, 40, 1722–1760; (c) Zhu, Y.; Wang, Q.; Cornwall, R. G.; Shi, Y. Organocatalytic Asymmetric Epoxidation and Aziridination of Olefins and Their Synthetic Applications. Chem. Rev. 2014, 114, 8199–8256; (d) Wang, C.; Yamamoto, H. Asymmetric Epoxidation Using Hydrogen Peroxide as Oxidant. Chem. Asian J. 2015, 10, 2056–2068; (e) Bryliakov, K. P. Catalytic Asymmetric Oxygenations with the Environmentally Benign Oxidants H2O2 and O2. Chem. Rev. 2017, 117, 11406–11459.
- 16 Colladon, M.; Scarso, A.; Sgarbossa, P.; Michelin, R. A.; Strukul, G. Asymmetric Epoxidation of Terminal Alkenes with Hydrogen Peroxide Catalyzed by Pentafluorophenyl PtII Complexes. J. Am. Chem. Soc. 2006, 128, 14006–14007.
- 17 Matsumoto, K.; Sawada, Y.; Saito, B.; Sakai, K.; Katsuki, T. Construction of Pseudo-Heterochiral and Homochiral Di-μ-oxotitanium(Schiff base) Dimers and Enantioselective Epoxidation Using Aqueous Hydrogen Peroxide. Angew. Chem. Int. Ed. 2005, 44, 4935–4939.
- 18 Wang, Q.; Neudörfl, J.-M.; Berkessel, A. Titanium cis-1,2-Diaminocyclohexane (cis-DACH) Salalen Catalysts for the Asymmetric Epoxidation of Terminal Non-Conjugated Olefins with Hydrogen Peroxide. Chem. Eur. J. 2015, 21, 247–254.
- 19 Lansing, M.; Engler, H.; Leuther, T. M.; Neudörfl, J.-M.; Berkessel, A. Titanium cis-1,2-Diaminocyclohexane Salalen Catalysts of Outstanding Activity and Enantioselectivity for the Asymmetric Epoxidation of Nonconjugated Terminal Olefins with Hydrogen Peroxide. ChemCatChem 2016, 8, 3706–3709.
- 20 Shen, D.; Qiu, B.; Xu, D.; Miao, C.; Xia, C.; Sun, W. Enantioselective Epoxidation of Olefins with H2O2 Catalyzed by Bioinspired Aminopyridine Manganese Complexes. Org. Lett. 2016, 18, 372−375.
- 21(a) Murphy, A.; Dubois, G.; Stack, T. D. P. Efficient Epoxidation of Electron-Deficient Olefins with a Cationic Manganese Complex. J. Am. Chem. Soc. 2003, 125, 5250; (b) Wu, M.; Wang, B.; Wang, S.; Xia, C.; Sun, W. Asymmetric Epoxidation of Olefins with Chiral Bioinspired Manganese Complexes. Org. Lett. 2009, 11, 3622
- 22(a) De Mayo, P.; Takashita, H.; Satter, A. B. M. A. The PhotoChemical Synthesis of 1,5-Diketones and Their Cyclisation: A New Annulation Process. Proc. Chem. Soc. 1962, 119; (b) De Mayo, P. Enone Photoannelation. Acc. Chem. Res. 1971, 4, 41−47.
- 23(a) Genzink, M. J.; Kidd, J. B.; Swords, W. B.; Yoon, T. P. Chiral Photocatalyst Structures in Asymmetric Photochemical Synthesis. Chem. Rev. 2022, 122, 1654−1716; (b) Großkopf, J.; Kratz, T.; Rigotti, T.; Bach, T. Enantioselective Photochemical Reactions Enabled by Triplet Energy Transfer. Chem. Rev. 2022, 122, 1626−1653.
- 24 Zhang, W.; Zhang, L.; Luo, S. Catalytic Asymmetric Visible-Light de Mayo Reaction by ZrCl4-Chiral Phosphoric Acid Complex. J. Am. Chem. Soc. 2023, 145, 14227−14232.
- 25 Kato, K.; Hirano, K.; Miura, M. Copper-Catalyzed Regio- and Enantioselective Aminoboration of Unactivated Terminal Alkenes. Chem. Eur. J. 2018, 24, 5775–5778.
- 26(a) Jana, R.; Pathak, T. P.; Sigman, M. S. Advances in Transition Metal (Pd,Ni,Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492; (b) Geist, E.; Kirschning, A.; Schmidta, T. sp3-sp3 Coupling Reactions in the Synthesis of Natural Products and Biologically Active Molecules. Nat. Prod. Rep. 2014, 31, 441-448; (c) Tollefson, E. J.; Hanna, L. E.; Jarvo, E. R. Stereospecific Nickel-Catalyzed Cross-Coupling Reactions of Benzylic Ethers and Esters. Acc. Chem. Res. 2015, 48, 2344–2353; (d) Tellis, J. C.; Kelly, C. B.; Primer, D. N.; Jouffroy, M.; Patel, N. R.; Molander, G. A. Single-Electron Transmetalation via Photoredox/ Nickel Dual Catalysis: Unlocking a New Paradigm for sp3–sp2 Cross-Coupling. Acc. Chem. Res. 2016, 49, 1429–1439; (e) Pan, Q.; Ping, Y.; Kong, W. Nickel-Catalyzed Ligand-Controlled Selective Reductive Cyclization/Cross-Couplings. Acc. Chem. Res. 2023, 56, 515–535.
- 27 Logan, K. M.; Sardini, S. R.; White, S. D.; Brown, M. K. Nickel-catalyzed Stereoselective Arylboration of Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 159–162; (b) Chen, L.-A.; Lear, A. R.; Gao, P.; Brown, M. K. Nickel-catalyzed Arylboration of Alkenylarenes: Synthesis of Boron Substituted Quaternary Carbons and Regiodivergent Reactions. Angew. Chem. Int. Ed. 2019, 58, 10956–10960.
- 28(a) Ye, Y.; Liu, J.; Xu, B.; Jiang, S.; Bai, R.; Li, S.; Xie, T.; Ye, X.-Y. Nickel-catalyzed Enantioselective 1,2-Vinylboration of Styrenes. Chem. Sci. 2021, 12, 13209–13215; (b) Duan, M.; Wang, Y.; Zhu, S. Nickel-catalyzed Asymmetric 1,2-Alkynylboration of Vinylarenes. Tetrahedron Lett. 2023, 114, 154247.
- 29(a) Chen, Z.; Brookhart, M. Exploring Ethylene/Polar Vinyl Monomer Copolymerizations Using Ni and Pd α-Diimine Catalysts. Acc. Chem. Res. 2018, 51, 1831–1839; (b) Li, Y.; Wu, D.; Cheng, H.-G.; Yin, G. Difunctionalization of Alkenes Involving Metal Migration. Angew. Chem. Int. Ed. 2020, 59, 7990–8003.
- 30 Li, Y.; Pang, H.; Wu, D.; Li, Z.; Wang, W.; Wei, H.; Fu, Y.; Yin, G. Nickel-catalyzed 1,1-Alkylboration of Electronically Unbiased Terminal Alkenes. Angew. Chem. Int. Ed. 2019, 58, 8872–8876.
- 31 Wang, W.; Ding, C.; Yin, G. Catalyst-controlled Enantioselective 1,1-Arylboration of Unactivated Olefins. Nat. Catal. 2020, 3, 951–958.
- 32 Sun, C.; Li, Y.; Yin, G. Practical Synthesis of Chiral Allylboronates by Asymmetric 1,1-Difunctionalization of Terminal Alkenes. Angew. Chem. Int. Ed. 2022, 61, e202209076.
- 33 Li, Z.; Shi, H.; Chen, X.; Peng, L.; Li, Y.; Yin, G. Nickel-Catalyzed Regio- and Enantioselective Borylative Coupling of Terminal Alkenes with Alkyl Halides Enabled by an Anionic Bisoxazoline Ligand. J. Am. Chem. Soc. 2023, 145, 13603−13614.
- 34(a) Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2013, 30, 849–868; (b) Miaskiewicz, S.; Reed, J. H.; Donets, P. A.; Oliveira, C. C.; Cramer, N. Chiral 1,3,2-Diazaphospholenes as Catalytic Molecular Hydrides for Enantioselective Conjugate Reductions. Angew. Chem. Int. Ed. 2018, 57, 4039–4042.
- 35(a) Newton, C. G.; Wang, S.-G.; Oliveira, C. C.; Cramer, N. Catalytic Enantioselective Transformations Involving C–H Bond Cleavage by Transition-Metal Complexes. Chem. Rev. 2017, 117, 8908–8976; (b) Zhang, Q.; Shi, B.-F. 2-(Pyridin-2-yl)isopropyl (PIP) Amine: An Enabling Directing Group for Divergent and Asymmetric Functionalization of Unactivated Methylene C(sp3)–H Bonds. Acc. Chem. Res. 2021, 54, 2750–2763; (c) Liu, C.-X.; Zhang, W.-W.; Yin, S.-Y.; Gu, Q.; You, S.-L. Synthesis of Atropisomers by Transition-Metal-Catalyzed Asymmetric C–H Functionalization Reactions. J. Am. Chem. Soc. 2021, 143, 14025–14040.
- 36 Trifonova, E. A.; Ankudinov, N. M.; Mikhaylov, A. A.; Chusov, D. A.; Nelyubina, Y. V.; Perekalin, D. S. A Planar-Chiral Rhodium(III) Catalyst with a Sterically Demanding Cyclopentadienyl Ligand and Its Application in the Enantioselective Synthesis of Dihydroisoquinolones. Angew. Chem. Int. Ed. 2018, 57, 7714–7718.
- 37 Ozols, K.; Jang, Y. S.; Cramer, N. Chiral Cyclopentadienyl Cobalt(III) Complexes Enable Highly Enantioselective 3d-Metal-Catalyzed C−H Functionalizations. J. Am. Chem. Soc. 2019, 141, 5675–5680.
- 38(a) Yan, M.; Kawamata, Y.; Baran, P. S. Synthetic Organic Electrochemical Methods Since 2000: On the Verge of a Renaissance. Chem. Rev. 2017, 117, 13230–13319; (b) Dwivedi, V.; Kalsi, D.; Sundararaju, B. Electrochemical-/Photoredox Aspects of Transition Metal-Catalyzed Directed C−H Bond Activation. ChemCatChem 2019, 11, 5160–5187; (c) Ackermann, L. Metalla-electrocatalyzed C–H Activation by Earth-Abundant 3d Metals and Beyond. Acc. Chem. Res. 2020, 53, 84–104.
- 39Yao, Q.-J.; Huang, F.-R.;. Chen, J.-H.; Zhong, M.-Y.; Shi, B.-F. Enantio- and Regioselective Electrooxidative Cobalt-Catalyzed C–H/N–H Annulation with Alkenes. Angew. Chem. Int. Ed. 2023, 62, e202218533.
- 40(a) Donohoe, T. J.; Callens, C. K. A.; Flores, A.; Lacy, A. R.; Rathi, A. H. Recent Developments in Methodology for the Direct Oxyamination of Olefins. Chem. Eur. J. 2011, 17, 58–76; (b) Song, Z.-L.; Fan, C.-A.; Tu, Y.-Q. Semipinacol Rearrangement in Natural Product Synthesis. Chem. Rev. 2011, 111, 7523–7556; (c) Casiraghi, G.; Zanardi, F.; Rassu, G.; Spanu, P. Stereoselective Approaches to Bioactive Carbohydrates and Alkaloids—with A Focus on Recent Syntheses Drawing from the Chiral Pool. Chem. Rev. 1995, 95, 1677–1716.
- 41(a) Hentges, S. G.; Sharpless, K. B. Asymmetric Induction in the Reaction of Osmium Tetroxide with Olefins. J. Am. Chem. Soc. 1980, 102, 4263–4265; (b) Kolb, H. C.; Van Nieuwenhze, M. S.; Sharpless, K. B. Catalytic Asymmetric Dihydroxylation. Chem. Rev. 1994, 94, 2483–2547.
- 42(a) Plietker, B. Selectivity versus Reactivity - Recent Advances in RuO4-Catalyzed Oxidations. Synthesis 2005, 2453–2472; (b) Yip, W.-P.; Yu, W.-Y.; Zhu, N.; Che, C.-M. Alkene cis-Dihydroxylation by [(Me3tacn)(CF3CO2)RuVIO2]ClO4 (Me3tacn = 1,4,7-Trimethyl-1,4,7- triazacyclononane): Structural Characterization of [3 + 2] Cycloadducts and Kinetic Studies. J. Am. Chem. Soc. 2005, 127, 14239–14249; (c) Yip, W.-P.; Ho, C.-M.; Zhu, N.; Lau, T.-C.; Che, C.-M. Homogeneous [RuIII(Me3tacn)Cl3]-Catalyzed Alkene cis-Dihydroxylation with Aqueous Hydrogen Peroxide. Chem. Asian J. 2008, 3, 70–77; (d) Neisius, N. M.; Plietker, B. Diastereoselective Ru-Catalyzed Cross-Metathesis− Dihydroxylation Sequence. An Efficient Approach toward Enantiomerically Enriched syn-Diols. J. Org. Chem. 2008, 73, 3218–3227.
- 43(a) Saisaha, P.; Pijper, D.; van Summeren, R. P.; Hoen, R.; Smit, C.; de Boer, J. W.; Hage, R.; Alsters, P. L.; Feringa, B. L.; Browne, W. R. Manganese Catalyzed cis-Dihydroxylation of Electron Deficient Alkenes with H2O2. Org. Biomol. Chem. 2010, 8, 4444–4450; (b) Wang, C.; Zong, L.; Tan, C.-H. Enantioselective Oxidation of Alkenes with Potassium Permanganate Catalyzed by Chiral Dicationic Bisguanidinium. J. Am. Chem. Soc. 2015, 137, 10677–10682.
- 44(a) Bruijnincx, P. C. A.; van Koten, G.; Klein Gebbink, R. J. M. Mononuclear Non-heme Iron Enzymes with the 2-His-1-carboxylate Facial Triad: Recent Developments in Enzymology and Modeling Studies. Chem. Soc. Rev. 2008, 37, 2716–2744; (b) Chow, T. W.-S.; Wong, E. L.-M.; Guo, Z.; Liu, Y.; Huang, J.-S.; Che, C.-M. cis-Dihydroxylation of Alkenes with Oxone Catalyzed by Iron Complexes of a Macrocyclic Tetraaza Ligand and Reaction Mechanism by ESI-MS Spectrometry and DFT Calculations. J. Am. Chem. Soc. 2010, 132, 13229–13239; (c) Prat, I.; Font, D.; Company, A.; Junge, K.; Ribas, X.; Beller, M.; Costas, M. Fe(PyTACN)-Catalyzed cis-Dihydroxylation of Olefins with Hydrogen Peroxide. Adv. Synth. Catal. 2013, 355, 947–956.
- 45 Zang, C.; Liu, Y.; Xu, Z.-J.; Tse, C.-W.; Guan, X.; Wei, J.; Huang, J.-S.; Che, C.-M. Highly Enantioselective Iron-Catalyzed cis-Dihydroxylation of Alkenes with Hydrogen Peroxide Oxidant via an FeIII-OOH Reactive Intermediate. Angew. Chem. Int. Ed. 2016, 55, 10253–10257.
- 46 Tian, B.; Chen, P.; Leng, X.; Liu, G. Palladium-catalysed Enantioselective Diacetoxylation of Terminal Alkenes. Nat. Catal. 2021, 4, 172–179.
- 47 Tian, B.; Li, X.; Chen, P.; Liu, G. Asymmetric Palladium-Catalyzed Oxycarbonylation of Terminal Alkenes: Efficient Access to b-Hydroxy Alkylcarboxylic Acids. Angew. Chem. Int. Ed. 2021, 60, 14881–14886.
- 48(a) Quirk, J.; Thornton, M.; Kirkpatrick, P. Rosuvastatin calcium. Nat. Rev. Drug Discovery 2003, 2, 769–770; (b) Uchida, R.; Kondo, A.; Yagi, A.; Nonaka, K.; Masuma, R.; Kobayashi, K.; Tomoda, H. Simpotentin, a New Potentiator of Amphotericin B Activity Against Candida Albicans, Produced by Simplicillium Minatense FKI-4981. J. Antibiot. 2019, 72, 134–140.
- 49 Yang, X.; Li, X.; Chen, P.; Liu, G. Palladium(II)-Catalyzed Enantioselective Hydrooxygenation of Unactivated Terminal Alkenes. J. Am. Chem. Soc. 2022, 144, 7972−7977.
- 50(a) Karjalainen, O. K.; Koskinen, A. M. P. Diastereoselective Synthesis of Vicinal Amino Alcohols. Org. Biomol. Chem. 2012, 10, 4311−4326; (b) Liu, S.; Zhang, B.-Q.; Xiao, W.-Y.; Li, Y.-L.; Deng, J. Recent Advances in Catalytic Asymmetric Syntheses of Functionalized Heterocycles via Halogenation/Chalcogenation of Carbon-Carbon Unsaturated Bonds. Adv. Synth. Catal. 2022, 364, 3974–4005.
- 51 Wata, C.; Hashimoto, T. Organoiodine-Catalyzed Enantioselective Intermolecular Oxyamination of Alkenes. J. Am. Chem. Soc. 2021, 143, 1745−1751.
- 52(a) Brown, H. C.; Singaram, B. The Development of A Simple General Procedure for Synthesis of Pure Enantiomers via Chiral Organoboranes. Acc. Chem. Res. 1988, 21, 287–293; (b) Leonori, D.; Aggarwal, V. K. Stereospecific Couplings of Secondary and Tertiary Boronic Esters. Angew. Chem. Int. Ed. 2015, 54, 1082–1096.
- 53 Brown, H. C.; Zweifel, G. Hydroboration as A Convenient Procedure for the Asymmetric Synthesis of Alcohols of High Optical Purity. J. Am. Chem. Soc. 1961, 83, 486–486.
- 54 Fan, W.; Li, L.; Zhang, G. Branched-Selective Alkene Hydroboration Catalyzed by Earth-Abundant Metals. J. Org. Chem. 2019, 84, 5987–5996.
- 55 Smith, J. R.; Collins, B. S. L.; Hesse, M. J.; Graham, M. A.; Myers, E. L.; Aggarwal, V. K. Enantioselective Rhodium(III)-Catalyzed Markovnikov Hydroboration of Unactivated Terminal Alkenes. J. Am. Chem. Soc. 2017, 139, 9148−9151.
- 56 Cai, Y.; Yang, X.-T.; Zhang, S.-Q.; Li, F.; Li, Y.-Q.; Ruan, L.-X.; Hong, X.; Shi, S.-L. Copper-Catalyzed Enantioselective Markovnikov Protoboration of α-Olefins Enabled by a Buttressed N–Heterocyclic Carbene Ligand. Angew. Chem. Int. Ed. 2018, 57, 1376–1380.
- 57 Cai, Y.; Shi, S.-L. ANIPE-Cu Catalyst Enables Highly Enantioselective Markovnikov Hydroboration of ±α-Olefins. Synlett 2021, 32, 545–550.
- 58(a) Grimme, S. Density Functional Theory with London Dispersion Corrections. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 211–228; (b) Yoshimura, T.; Maeda, S.; Taketsugu, T.; Sawamura, M.; Morokuma, K.; Mori, S. Exploring the Full Catalytic Cycle of Rhodium(I)–BINAP Catalysed Isomerisation of Allylic Amines: A Graph Theory Approach for Path Optimisation. Chem. Sci. 2017, 8, 4475–4488; (c) Guan, Y.; Wheeler, S. E. Automated Quantum Mechanical Predictions of Enantioselectivity in A Rhodium-catalyzed Asymmetric Hydrogenation. Angew. Chem. Int. Ed. 2017, 56, 9101–9105.
- 59(a) Milo, A.; Neel, A. J.; Toste, F. D.; Sigman, M. S. A Data-intensive Approach to Mechanistic Elucidation Applied to Chiral Anion Catalysis. Science 2015, 347, 737–743; (b) Sigman, M. S.; Harper, K. C.; Bess, E. N.; Milo, A. The Development of Multidimensional Analysis Tools for Asymmetric Catalysis and Beyond. Acc. Chem. Res. 2016, 49, 1292–1301.
- 60 Iwamoto, H.; Imamoto, T.; Ito, H. Computational Design of High- performance Ligand for Enantioselective Markovnikov Hydroboration of Aliphatic Terminal Alkenes. Nat. Commun. 2018, 9, 2290.
- 61 Aldhous, T. P.; Chung, R. W. M.; Dalling, A. G.; Bower, J. F. Enantioselective Intermolecular Murai-Type Alkene Hydroarylation Reactions. Synthesis 2021, 53, 2961–2975.
- 62 Grélaud, S.; Cooper, P.; Feron, L. J.; Bower, J. F. Branch-Selective and Enantioselective Iridium-Catalyzed Alkene Hydroarylation via Anilide-Directed C−H Oxidative Addition. J. Am. Chem. Soc. 2018, 140, 9351−9356.
- 63 Pesciaioli, F.; Dhawa, U.; Oliveira, J. C. A.; Yin, R.; John, M.; Ackermann, L. Enantioselective Cobalt(III)-Catalyzed C–H Activation Enabled by Chiral Carboxylic Acid Cooperation. Angew. Chem. Int. Ed. 2018, 57, 15425–15429.
- 64 Liu, Y.-H.; Xie, P.-P.; Liu, L.; Fan, J.; Zhang, Z.-Z.; Hong, X.; Shi, B.-F. Cp*Co(III)-Catalyzed Enantioselective Hydroarylation of Unactivated Terminal Alkenes via C−H Activation. J. Am. Chem. Soc. 2021, 143, 19112−19120.
- 65(a) Kakiuchi, F.; Tanaka, Y.; Sato, T.; Chatani, N.; Murai, S. Catalytic Addition of Olefinic C−H Bonds to Olefins. Chem. Lett. 1995, 24, 679−680; (b) Hirano, M. Recent Advances in the Catalytic Linear Cross-Dimerizations. ACS Catal. 2019, 9, 1408−1430; (c) Rajanbabu, T. V. In Pursuit of an Ideal C–C Bond-Forming Reaction: Development and Applications of the Hydrovinylation of Olefins. Synlett 2009, 2009, 853−885.
- 66(a) Ho, C.-Y.; Chan, C.-W.; He, L. Catalytic Asymmetric Hydroalkenylation of Vinylarenes: Electronic Effects of Substrates and Chiral N–Heterocyclic Carbene Ligands. Angew. Chem. Int. Ed. 2015, 54, 4512−4516; (b) Hirano, M. Recent Advances in the Catalytic Linear Cross-Dimerizations. ACS Catal. 2019, 9, 1408−1430; (c) Chen, Y.; Dang, L.; Ho, C. Y. NHC–Ni Catalyzed Enantioselective Synthesis of 1,4-Dienes by Cross-hydroalkenylation of Cyclic 1,3-Dienes and Heterosubstituted Terminal Olefins. Nat. Commun. 2020, 11, 2269.
- 67 Sun, X.; Lin, E.-Z.; Li, B.-J. Iridium-Catalyzed Branch-Selective and Enantioselective Hydroalkenylation of α-Olefins through C−H Cleavage of Enamides. J. Am. Chem. Soc. 2022, 144, 17351−17358
- 68(a) Alkaloids: A Treasury of Poisons and Medicines, Eds.: Funayama, S.; Cordell, G. A., Waltham, MA, 2014;
(b) Brown, B. R. The Organic Chemistry of Aliphatic Nitrogen Compounds, Oxford University, New York, 1994;
(c) Nugent, T. C. Chiral Amine Synthesis: Methods, Developments and Applications, Wiley-VCH, 2010.
10.1002/9783527629541 Google Scholar
- 69(a) Zhang, Z.; Lee, S. D.; Widenhoefer, R. A. Intermolecular Hydroamination of Ethylene and 1-Alkenes with Cyclic Ureas Catalyzed by Achiral and Chiral Gold(I) Complexes. J. Am. Chem. Soc. 2009, 131, 5372–5373; (b) Reznichenko, A. L.; Nguyen, H. N.; Hultzsch, K. C. Asymmetric Intermolecular Hydroamination of Unactivated Alkenes with Simple Amines. Angew. Chem. Int. Ed. 2010, 49, 8984 –8987; (c) Pan, S.; Endo, K.; Shibata, T. Ir(I)-Catalyzed Intermolecular Regio- and Enantioselective Hydroamination of Alkenes with Heteroaromatic Amines. Org. Lett. 2012, 14, 780-783; (d) Sevov, C. S.; Zhou, J. S.; Hartwig, J. F. Iridium-Catalyzed, Intermolecular Hydroamination of Unactivated Alkenes with Indoles. J. Am. Chem. Soc. 2014, 136, 3200−3207.
- 70 Ma, S.; Xi, Y.; Fan, H.; Roediger, S.; Hartwig, J. F. Enantioselective Hydroamination of Unactivated Terminal Alkenes. Chem 2022, 8, 532–542.
- 71(a) Gunanathan, C.; Milstein, D. Applications of Acceptorless Dehydrogenation and Related Transformations in Chemical Synthesis. Science 2013, 341, 1229712; (b) Chen, B.; Wang, L.; Gao, S. Recent Advances in Aerobic Oxidation of Alcohols and Amines to Imines. ACS Catal. 2015, 5, 5851–5876; (c) Hazra, S.; Malik, E.; Nair, A.; Tiwari, V.; Dolui, P.; Elias, A. J. Catalytic Oxidation of Alcohols and Amines to Value-Added Chemicals using Water as the Solvent. Chem. Asian J. 2020, 15, 1916–1936.
- 72(a) Silicon in Organic, Organometallic, and Polymer Chemistry, Ed.: Brook, M. A., Wiley, New York, 1999; (b) Franz, A. K.; Wilson, S. O. Organosilicon Molecules with Medicinal Applications. J. Med. Chem. 2013, 56, 388–405; (c) Min, G. K.; Hernández, D.; Skrydstrup, T. Efficient Routes to Carbon–Silicon Bond Formation for the Synthesis of Silicon-Containing Peptides and Azasilaheterocycles. Acc. Chem. Res. 2013, 46, 457–470; (d) Zhang, H.-J.; Priebbenow, D. L.; Bolm, C. Acylsilanes: Valuable Organosilicon Reagents in Organic Synthesis. Chem. Soc. Rev. 2013, 42, 8540–8571; (e) Denmark, S. E.; Ambrosi, A. Why You Really Should Consider Using Palladium-Catalyzed Cross- Coupling of Silanols and Silanolates. Org. Process Res. Dev. 2015, 19, 982–994; (f) Komiyama, T.; Minami, Y.; Hiyama, T. Recent Advances in Transition-Metal-Catalyzed Synthetic Transformations of Organosilicon Reagents. ACS Catal. 2017, 7, 631–651.
- 73(a) Shintani, R. Recent Advances in the Transition-Metal-Catalyzed Enantioselective Synthesis of Silicon-Stereogenic Organosilanes. Asian J. Org. Chem. 2015, 4, 510–614; (b) Bauer, J. O.; Strohmann, C. Recent Progress in Asymmetric Synthesis and Application of Difunctionalized Silicon-Stereogenic Silanes. Eur. J. Inorg. Chem. 2016, 2016, 2868–2881.
- 74(a) Zhang, Y.-Z.; Zhu, S.-F.; Wang, L.-X.; Zhou, Q.-L. Copper-Catalyzed Highly Enantioselective Carbenoid Insertion into Si-H Bonds. Angew. Chem. Int. Ed. 2008, 47, 8496–8498; (b) Chen, D.; Zhu, D.-X.; Xu, M.-H. Rhodium(I)-Catalyzed Highly Enantioselective Insertion of Carbenoid into Si–H: Efficient Access to Functional Chiral Silanes. J. Am. Chem. Soc. 2016, 138, 1498–1501.
- 75(a) Zhang, Y.-Z.; Zhu, S.-F.; Wang, L.-X.; Zhou, Q.-L. Copper-Catalyzed Highly Enantioselective Carbenoid Insertion into Si-H Bonds. Angew. Chem. 2008, 120, 8624–8626;
10.1002/ange.200803192 Google Scholar(b) Kan, S. B. J.; Lewis, R. D.; Chen, K.; Arnold, F. H. Directed Evolution of Cytochrome c for Carbon–silicon Bond Formation: Bringing Silicon to Life. Science 2016, 354, 1048–1051; (c) Yang, L.-L.; Evans, D.; Xu, B.; Li, W.-T.; Li, M.-L.; Zhu, S.-F.; Houk, K. N.; Zhou, Q.-L. Enantioselective Diarylcarbene Insertion into Si–H Bonds Induced by Electronic Properties of the Carbenes. J. Am. Chem. Soc. 2020, 142, 12394–12399; (d) Yang, L.-L.; Ouyang, J.; Zou, H.-N.; Zhu, S.-F.; Zhou, Q.-L. Enantioselective Insertion of Alkynyl Carbenes into Si–H Bonds: An Efficient Access to Chiral Propargylsilanes and Allenylsilanes. J. Am. Chem. Soc. 2021, 143, 6401–6406; (e) Garcia-Borràs, M.; Kan, S. B. J.; Lewis, R. D.; Tang, A.; Jimenez-Osés, G.; Arnold, F. H.; Houk, K. N. Origin and Control of Chemoselectivity in Cytochrome c Catalyzed Carbene Transfer into Si–H and N–H bonds. J. Am. Chem. Soc. 2021, 143, 7114–7123.
- 76(a) Gibson, S. E.; Rudd, M. The Role of Secondary Interactions in the Asymmetric Palladium-Catalysed Hydrosilylation of Olefins with Monophosphane Ligands. Adv. Synth. Catal. 2007, 349, 781–795; (b) Ito, J.-i.; Nishiyama, H. Bifunctional Phebox Complexes for Asymmetric Catalysis. Top. Organomet. Chem. 2011, 37, 185–205.
- 77(a) Hydrosilylation: A Comprehensive Review on Recent Advances, Ed.: Marciniec, B., Springer, Berlin, 2009, Vol. 1, Part 1; (b) Nakajima, Y.; Shimada, S. Hydrosilylation reaction of olefins: recent advances and perspectives. RSC Adv. 2015, 5, 20603–20616; (c) Du, X.-Y.; Huang, Z. Advances in Base-Metal-Catalyzed Alkene Hydrosilylation. ACS Catal. 2017, 7, 1227–1243; (d) Fu, P.-F.; Brard, L.; Li, Y.-W.; Marks, T. J. Regioselection and Enantioselection in Organolanthanide-Catalyzed Olefin Hydrosilylation. A Kinetic and Mechanistic Study. J. Am. Chem. Soc. 1995, 117, 7157–7168.
- 78 Gribble Jr., M. W.; Pirnot, M. T.; Bandar, J. S.; Liu, R. Y.; Buchwald, S. L. Asymmetric Copper Hydride-Catalyzed Markovnikov Hydrosilylation of Vinylarenes and Vinyl Heterocycles. J. Am. Chem. Soc. 2017, 139, 2192–2195.
- 79 Cheng, B.; Lu, P.; Zhang, H.; Cheng, X.; Lu, Z. Highly Enantioselective Cobalt-Catalyzed Hydrosilylation of Alkenes. J. Am. Chem. Soc. 2017, 139, 9439−9442.
- 80 Cheng, B.; Liu, W.; Lu, Z. Iron-Catalyzed Highly Enantioselective Hydrosilylation of Unactivated Terminal Alkenes. J. Am. Chem. Soc. 2018, 140, 5014−5017.
- 81(a) Boersma, A. J.; Coquiére, D.; Geerdink, D.; Rosati, F.; Feringa, B. L.; Roelfes, G. Catalytic Enantioselective Syn Hydration of Enones in Water Using a DNA-based Catalyst. Nat. Chem. 2010, 2, 991–995; (b) Wuensch, C.; Gross, J.; Steinkellner, G.; Gruber, K.; Glueck, S. M.; Faber, K. Asymmetric Enzymatic Hydration of Hydroxystyrene Derivatives. Angew. Chem. Int. Ed. 2013, 52, 2293–2297.
- 82(a) Hiseni, A.; Arends, I. W. C. E.; Otten, L. G. Biochemical Characterization of the Carotenoid 1,2-Hydratases (CrtC) from Rubrivivax Gelatinosus and Thiocapsa Roseopersicina. Appl. Microbiol. Biotechnol. 2011, 91, 1029–1036; (b) Hiseni, A.; Otten, L. G.; Arends, I. W. C. E. Identification of Catalytically Important Residues of the Carotenoid 1,2-Hydratases from Rubrivivax Gelatinosus and Thiocapsa Roseopersicina. Appl. Microbiol. Biotechnol. 2016, 100, 1275–1284; (c) Weidenweber, S.; Marmulla, R.; Ermler, U.; Harder, J. X-ray Structure of Linalool Dehydratase/isomerase from Castellaniella Defragrans Reveals Enzymatic Alkene Synthesis. FEBS Lett. 2016, 590, 1375–1383; (d) Nestl, B. M.; Geinitz, C.; Popa, S.; Rizek, S.; Haselbeck, R. J.; Stephen, R.; Noble, M. A.; Fischer, M.-P.; Ralph, E. C.; Hau, H. T.; Man, H.; Omar, M.; Turkenburg, J. P.; van Dien, S.; Culler, S. J.; Grogan, G.; Hauer, B. Structural and Functional Insights into Asymmetric Enzymatic Dehydration of Alkenols. Nat. Chem. Biol. 2017, 13, 275–281; (e) Lorenzen, J.; Janke, R.; Waldow, A.; Qoura, F.; Loll, B.; Brgck, T. Rhodococcus Erythropolis Oleate Hydratase: a New Member in the Oleate Hydratase Family Tree—Biochemical and Structural Studies. ChemCatChem 2018, 10, 407–414.
- 83 Demming, R. M.; Hammer, S. C.; Nestl, B. M.; Gergel, S.; Fademrecht, S.; Pleiss, J.; Hauer, B. Asymmetric Enzymatic Hydration of Unactivated, Aliphatic Alkenes. Angew. Chem. Int. Ed. 2019, 58, 173–177.
- 84 Kato, K.; Mukaiyama, T. Iron(III) Complex Catalyzed Nitrosation of Terminal and 1,2-Disubstituted Olefins with Butyl Nitrite and Phenylsilane. Chem. Lett. 1992, 21, 1137–1140.
- 85(a) Waser, J.; Carreira, E. M. Convenient Synthesis of Alkylhydrazides by the Cobalt-catalyzed Hydrohydrazination Reaction of Olefins and Azodicarboxylates. J. Am. Chem. Soc. 2004, 126, 5676–5677; (b) Waser, J.; Gaspar, B.; Nambu, H.; Carreira, E. M. Hydrazines and Azides via the Metal-Catalyzed Hydrohydrazination and Hydroazidation of Olefins. J. Am. Chem. Soc. 2006, 128, 11693–11712; (c) Waser, J.; Carreira, E. M. Catalytic Hydrohydrazination of a Wide Range of Alkenes with a Simple Mn Complex. Angew. Chem. Int. Ed. 2004, 43, 4099–4102; (d) Zhang, Y.; Huang, C.; Lin, X.; Hu, Q.; Hu, B.; Zhou, Y.; Zhu, G. Modular Synthesis of Alkylarylazo Compounds via Iron(III)- catalyzed Olefin Hydroamination. Org. Lett. 2019, 21, 2261–2264; (e) Zhu, K.-L.; Shaver, M. P.; Thomas, S. P. Amine-Bis(phenolate) Iron(III)-catalyzed Formal Hydroamination of Olefins. Chem. Asian J. 2016, 11, 977–980; (f) Zheng, J.; Qi, J.-F.; Cui, S.-L. Fe-catalyzed Olefin Hydroamination with Diazo Compounds for Hydrazone Synthesis. Org. Lett. 2016, 18, 128–131; (g) Waser, J.; Nambu, H.; Carreira, E. M. Cobalt-catalyzed Hydroazidation of Olefins: Convenient Access to Alkyl Azides. J. Am. Chem. Soc. 2005, 127, 8294–8295.
- 86 Shen, X.; Chen, X.; Chen, J.; Sun, Y.; Cheng, Z.; Lu, Z. Ligand-promoted Cobalt-catalyzed Radical Hydroamination of Alkenes. Nat. Commun. 2020, 11, 783.
- 87 Green, S. A.; Crossley, S. W. M.; Matos, J. L. M.; Vásquez-Céspedes, S.; Shevick, S. L.; Shenvi, R. A. The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer. Acc. Chem. Res. 2018, 51, 2628–2640.
- 88 Chen, J.; Shen, X.; Lu, Z. Cobalt-Catalyzed Markovnikov Selective Sequential Hydrogenation/Hydrohydrazidation of Aliphatic Terminal Alkynes. J. Am. Chem. Soc. 2020, 142, 14455–14460.
- 89 Sun, Y.; Guo, J.; Shen, X.; Lu, Z. Ligand Relay Catalysis for Cobalt-catalyzed Sequential Hydrosilylation and Hydrohydrazidation of Terminal Alkynes. Nat. Commun. 2022, 13, 650.
- 90 Nie, Z.; Chiou, M.-F.; Cui, J.; Qu, Y.; Zhu, X.; Jian, W.; Xiong, H.; Li, Y.; Bao, H. Copper-Catalyzed Radical Enantioselective Carbo-Esterification of Styrenes Enabled by a Perfluoroalkylated-PyBox Ligand. Angew. Chem. Int. Ed. 2022, 61, e20220207.
- 91 Zhou, H.; Fan, L.-W.; Ren, Y.-Q.; Wang, L.-L.; Yang, C.-J.; Gu, Q.-S.; Li, Z.-L.; Liu, X.-Y. Copper-Catalyzed Chemo- and Enantioselective Radical 1,2-Carbophosphonylation of Styrenes. Angew. Chem. Int. Ed. 2023, 62, e202218523.