Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture
- Page: 805
- First Published: 15 March 2024

As classic molecular magnets with magnetic bistability, spin-crossover (SCO) compounds have potentially important application value in molecular electronics, sensing, and information storage. Multistable control of SCO compounds is more difficult than bistability control. In this paper, we propose a strategy to control the number of spin-transition steps to further understand the control of the multistability of SCO compounds. More details are discussed in the article by Li et al. on page 879—886.
Inside Cover Picture
Inside Cover Picture
- Page: 806
- First Published: 15 March 2024

Inspired by the skin of Osteichthyes fishes, a Janus hydrogel coating consisting of slippery and sticky layers is successfully prepared by a two-step UV light irradiation at room temperature. The slippery layer replicates the structure of cycloid scales, while the nature of hydrogel mimics the mucus on fish skin. The Janus hydrogel coating possesses prominent mechanical, anti-fouling and drag reduction properties. More details are discussed in the article by Xue et al. on page 867—872.
Contents
Concise Reports
Sarcocinerenoids A—J, Eight Rare Capnosane-Type and Two New Cage-Type Cembranoids with Promoting Angiogenesis Activity from the South China Sea Soft Coral Sarcophyton cinereum
- Pages: 815-822
- First Published: 07 December 2023
Nickel-Catalyzed Regio- and Stereoselective Defluorinative Arylation of gem-Difluorinated Cyclopropanes
- Pages: 823-828
- First Published: 07 December 2023
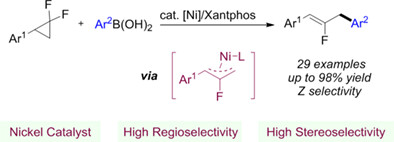
A nickel-catalyzed cross-coupling of gem-difluorinated cyclopropanes with boronic acids was reported, providing the corresponding arylated 2-fluoroallylic scaffolds with high regioselectivity and Z-stereoselectivity. Mechanistic studies proposed a Ni(II)-fluoroallyl pathway and clarified the origin of the high linear selectivity.
Palladium-Catalyzed [4+2] and [6+2] Dipolar Cycloadditions for the Construction of Benzo[d]isothiazole 1,1-Dioxide Fused 1,3-Oxazinanes and 1,3-Oxazocanes
- Pages: 829-834
- First Published: 07 December 2023
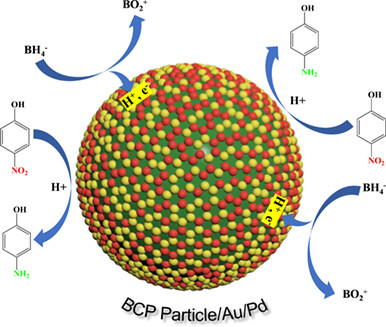
Controlled Synthesis of Metal-Nanoparticles Decorated Block Copolymer Hybrid Particles via Emulsion Confined Self-assembly and Their Catalytic Applications
- Pages: 835-840
- First Published: 11 December 2023
Practical Synthesis of Valbenazine via 1,3-Dipolar Cycloaddition
- Pages: 841-845
- First Published: 05 December 2023
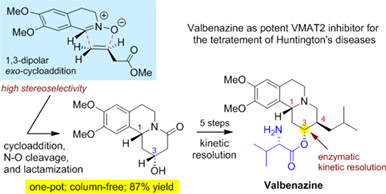
Valbenazine (Ingrezza), a potent compound that selectively inhibits VMAT2 through an active metabolite HTBZ, has been approved for the treatment of tardive dyskinesia and very recently for chorea associated with Huntington's disease. In this report, a practical synthesis of HTBZ and valbenazine has been achieved, featuring a highly stereoselective 1,3-dipolar cycloaddition and an enzymatic kinetic resolution. The cascade process toward pyrido[2,1-a]isoquinoline including cycloaddition, cleavage of the N—O bond, and lactamization proved to be operationally feasible. The allure of enzymatic resolution developed in this work provides a rapid access to THIQ-fused piperidine in various bioactive substances.
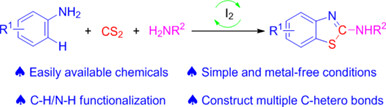
Metal-Free Synthesis of 2-Aminobenzothiazoles via I2-Catalyzed Tandem Cyclization Reaction of Amines and Carbon Disulfide
- Pages: 846-852
- First Published: 19 December 2023
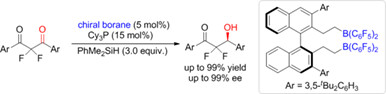
Asymmetric Partial Hydrosilylation of 2,2-Difluoro-1,3-diketones with Chiral Frustrated Lewis Pairs
- Pages: 853-857
- First Published: 13 December 2023
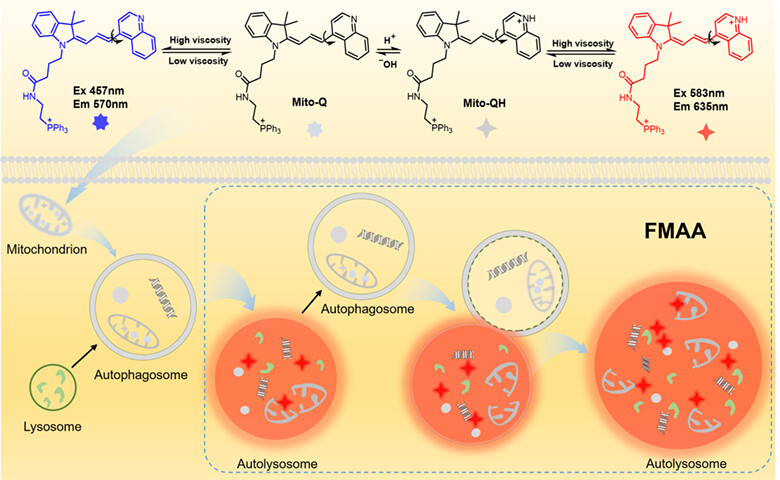
A High-Contrast Autolysosome Probe for Detecting Interaction between Autophagosomes and Autolysosomes in Mitophagy
- Pages: 858-866
- First Published: 19 December 2023
Fish Skin-Inspired Janus Hydrogel Coating for Drag Reduction
- Pages: 867-872
- First Published: 26 December 2023
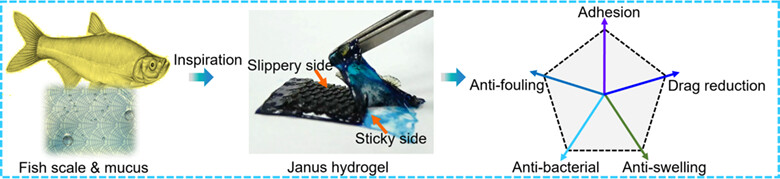
Inspired by the skin of Osteichthyes fishes, a Janus hydrogel coating consisting of slippery and sticky layers is successfully prepared by a two-step UV light irradiation at room temperature. The slippery layer replicates the structure of cycloid scales, while the nature of hydrogel mimics the mucus on fish skin. The Janus hydrogel coating possesses prominent mechanical, anti-fouling and drag reduction properties.
Breaking Report
Ni-Catalyzed Enantioselective Difunctionalization of Alkynes to Azepine Derivatives Bearing a Quaternary Center and an Unprotected Imine
- Pages: 873-878
- First Published: 26 December 2023

A Ni(II)-catalyzed asymmetric difunctionalization of alkynes is reported. This method involves intermolecular regioselective arylation of the alkynes and intramolecular desymmetrization of dinitriles, enabling the synthesis of enantioenriched azepine derivatives. The reaction exhibits good tolerance toward various functional groups, resulting in high yields and enantioselectivities.
Comprehensive Report
Controlling Three-Step and Five-Step Spin Transitions by Polymorphism in an FeIII Spin Crossover Complex
- Pages: 879-886
- First Published: 19 December 2023
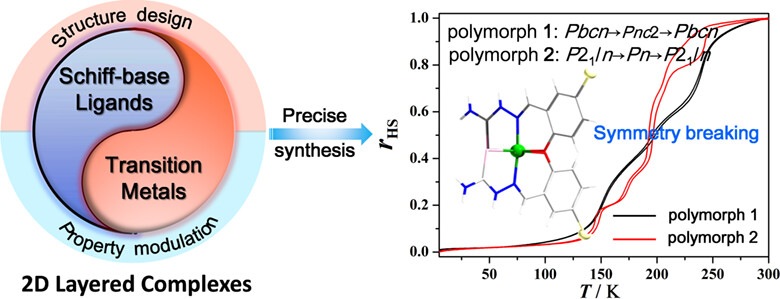
[FeIII(H-5-Br-thsa)(5-Br-thsa)]·H2O, which exists in two polymorphic forms that exhibit SCO, is reported. Polymorph 1 exhibits three-step SCO, while polymorph 2 exhibits five-step SCO, which effectively regulates multi-step SCO behavior. Polymorphs 1 and 2 crystallize in different space groups during the entire spin-transition process, and two-step symmetry breaking was observed (Pbcn → Pnc2 → Pbcn for polymorph 1; P21/n → Pn → P21/n for polymorph 2).
Critical Reviews
Synthesis of Organofluorine Compounds with Acylsilanes†
- Pages: 887-902
- First Published: 29 November 2023
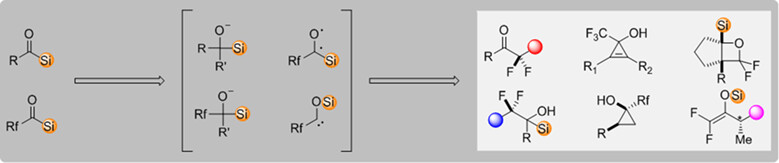
Organofluorine compounds are central in synthetic chemistry, medicinal chemistry and material chemistry. In this review, we summarize the investigations on the synthesis of organofluorine compounds with acylsilanes. For the non-fluorinated acylsilanes, the in situ generation of difluoroenoxysilanes from the reactions of the acylsilanes with trifluoromethylation reagents is the major pathway, leading to the facile preparation of various α,α-difluoroketones. For the fluoroalkylacylsilanes, apart from the in situ generation of difluoroenoxysilanes through anion Brook rearrangement, radical Brook rearrangement of the photoexcited acylsilanes and the selective control of the reactivities of the biradicals pave the way for the synthesis of a variety of organofluorine compounds. In general, most of these reactions gave racemic products, and the asymmetric synthesis of organofluorine compounds with acylsilanes is still rare, which would be a potential direction of this field.
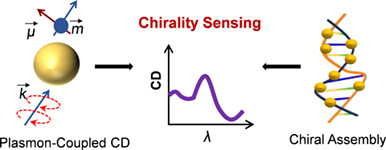
Rational Design of Plasmonic Nanoparticle-Molecule Complexes for Chirality Sensing†
- Pages: 903-919
- First Published: 27 November 2023
Meeting the New Members of the Chinese Academy of Sciences (Chemistry, 2023)
Meeting the New Members of the Chinese Academy of Sciences (Chemistry, 2023)
- Pages: 920-928
- First Published: 15 March 2024
Inside Back Cover
Inside Back Cover
- Page: 931
- First Published: 15 March 2024

Azepine ring is a prominent structural scaffold in biologically significant molecules. This study demonstrates a Ni(II)-catalyzed alkyne functionalization/cyclization cascade reaction of alkyne-tethered malononitriles, involving intermolecular regioselective arylation of the alkynes and desymmetrizing addition onto the nitrile group, to access azepine derivatives. This strategy introduces an all-carbon quaternary stereocenter and an unprotected imine functionality simultaneously to the azepine scaffold, showing great promise for subsequent transformations. More details are discussed in the article by Liu et al. on page 873—878.
Back Cover
Back Cover
- Page: 932
- First Published: 15 March 2024

2-Aminobenzothiazoles derivatives have revealed a broad spectrum of biological activities, such as anti-HIV, anti-inflammatory, antioxidant, anti-microbial, anti-tumour, anti-infective, and anti-convulsant activities. A great amount of 2-aminobenzothiazole derivatives have been applied in drugs for the treatment of human diseases. A convenient approach for the construction of 2-aminobenzothiazoles via I2-catalyzed tandem cyclization reaction of amines and carbon disulfide has been developed. More details are discussed in the article by Deng et al. on page 846—852.





![Palladium-Catalyzed [4+2] and [6+2] Dipolar Cycloadditions for the Construction of Benzo[d]isothiazole 1,1-Dioxide Fused 1,3-Oxazinanes and 1,3-Oxazocanes†](/cms/asset/6189fb0d-6564-4377-a859-6dbfadc197d0/cjoc202300668-toc-0001-m.jpg)