Nickel-Catalyzed Regio- and Stereoselective Defluorinative Arylation of gem-Difluorinated Cyclopropanes
Shutao Qi
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 China
The authors contribute equally.
Search for more papers by this authorYunkai Hua
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 China
The authors contribute equally.
Search for more papers by this authorLiangkai Pan
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 China
Search for more papers by this authorCorresponding Author
Junfeng Yang
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 China
Fudan Zhangjiang Institute, Shanghai, 201203 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Junliang Zhang
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorShutao Qi
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 China
The authors contribute equally.
Search for more papers by this authorYunkai Hua
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 China
The authors contribute equally.
Search for more papers by this authorLiangkai Pan
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 China
Search for more papers by this authorCorresponding Author
Junfeng Yang
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 China
Fudan Zhangjiang Institute, Shanghai, 201203 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Junliang Zhang
Department of Chemistry, Fudan University, 2005 Songhu Road, Shanghai, 200438 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
Herein, we report nickel-catalyzed cross-coupling of gem-difluorinated cyclopropanes with boronic acids, providing the corresponding arylated 2-fluoroallylic scaffolds. This approach used commercially available phosphine ligand Xantphos to obtain monofluorinated alkenes with high regioselectivity and Z-stereoselectivity. Mechanistic studies proposed a Ni(II)-fluoroallyl pathway and excluded the radical pathway. Meanwhile, DFT study of the reductive elimination clarified the origin of the high linear selectivity.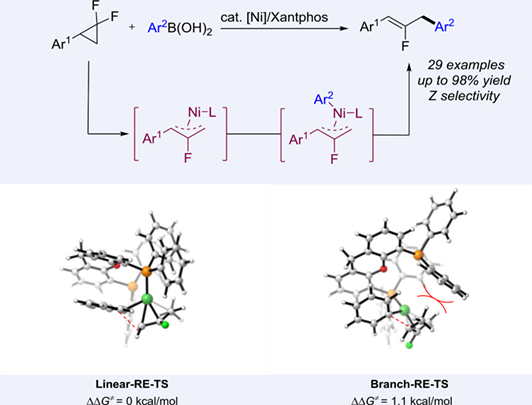
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300579-sup-0001-Supinfo.pdfPDF document, 7.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Kirsch, P., Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications, 2nd ed., Wiley-VCH, Weinheim, 2013;
10.1002/9783527651351 Google Scholar(b) Ojima, I., Fluorine in Medicinal Chemistry and Chemical Biology, Wiley, Chichester, 2009;10.1002/9781444312096 Google Scholar(c) Bƒguƒ, J. P.; Bonnet-Delpon, D., Bioorganic and Medicinal Chemistry of Fluorine, Wiley, Hoboken, 2008; (d) Fujita, T.; Fuchibe, K.; Ichikawa, J. Transition-Metal-Mediated and -Catalyzed C−F Bond Activation by Fluorine Elimination. Angew. Chem. Int. Ed. 2019, 58, 390–402; (e) Zhang, X.; Cao, S. Recent Advances in the Synthesis and C-F Functionalization of gem-Difluoroalkenes. Tetrahedron Lett. 2017, 58, 375–392; (f) Bartlett, P. A.; Otake, A. Fluoroalkenes as Peptide Isosteres: Ground State Analog Inhibitors of Thermolysin. J. Org. Chem. 1995, 60, 3107–3111; (g) Osada, S.; Sano, S.; Ueyama, M.; Chuman, Y.; Kodama, H.; Sakaguchi, K. Fluoroalkene Modification of Mercaptoacetamide-based Histone Deacetylase Inhibitors. Bioorg. Med. Chem. 2010, 18, 605–611; (h) Oishi, S.; Kamitani, H.; Kodama, E.; Matsuoka, M.; Fujii, N. Peptide Bond Mimicry by (E)-Alkene and (Z)-Fluoroalkene Peptide Isosteres: Synthesis and Bioevaluation of α-Helical Anti-HIV Peptide Analogues. Org. Biomol. Chem. 2009, 7, 2872–2877; (i) He, Y.-P.; Wu, H.; Wang, Q.; Zhu, J. Palladium-Catalyzed Enantioselective Cacchi Reaction: Asymmetric Synthesis of Axially Chiral 2,3-Disubstituted Indoles. Angew. Chem. Int. Ed. 2020, 59, 2105–2109.
- 2(a) McCune, C. D.; Beio, M. L.; Sturdivant, J. M.; Salud-Bea, R.; Darnell, B. M.; Berkowitz, D. B. Synthesis and Deployment of an Elusive Fluorovinyl Cation Equivalent: Access to Quaternary α-(1′-Fluoro) vinyl Amino Acids as Potential PLP Enzyme Inactivators. J. Am. Chem. Soc. 2017, 139, 14077–14089; (b) Pigeon, X.; Bergeron, M.; Barab, F.; Dub, P.; Frost, H. N.; Paquin, J. F. Activation of Allylic C-F bonds: Palladium-Catalyzed Allylic Amination of 3,3-Difluoropropenes. Angew. Chem. Int. Ed. 2010, 49, 1123–1127; (c) Bergeron, M.; Johnson, T.; Paquin, J. F. The Use of Fluoride as a Leaving Group: SN2’ Displacement of a C-F Bond on 3,3-Difluoropropenes with Organolithium Reagents to Give Direct Access to Monofluoroalkenes Angew. Chem. Int. Ed. 2011, 50, 11112–11116; (d) Narumi, T.; Tomita, K.; Inokuchi, E.; Kobayashi, K.; Oishi, S.; Ohno, H.; Fujii, N. Facile Synthesis of Fluoroalkenes by Palladium-Catalyzed Reductive Defluorination of Allylic gem-Difluorides Org. Lett. 2007, 9, 3465–3468.
- 3(a) Ichitsuka, T.; Fujita, T.; Arita, T.; Ichikawa, J. Double C–F Bond Activation through β-Fluorine Elimination: Nickel-Mediated [3+2] Cycloaddition of 2-Trifluoromethyl-1-alkenes with Alkynes Angew. Chem. Int. Ed. 2014, 53, 7564–7568; (b) Pinto, A.; Jia, Y.; Neuville, L.; Zhu, J. Palladium-Catalyzed Enantioselective Domino Heck-Cyanation Sequence: Development and Application to the Total Synthesis of Esermethole and Physostigmine. Chem. Eur. J. 2007, 13, 961–967.
- 4(a) Pigeon, X.; Bergeron, M.; Barab, F.; Dub, P.; Frost, H. N.; Paquin, J. F. Activation of Allylic C–F bonds: Palladium-Catalyzed Allylic Amination of 3,3-Difluoropropenes. Angew. Chem. Int. Ed. 2010, 49, 1123–1127; (b) Nihei, T.; Hoshino, T.; Konno, T. Unusual Reaction Behavior of gem-Difluorocyclopropane Derivatives: Stereoselective Synthesis of β-Monofluoroallylic Alcohols, Ethers, Esters, and Amide. Org. Lett. 2014, 16, 4170–4173.
- 5(a) Tian, P.; Feng, C.; Loh, T. P. Rhodium-catalysed C(sp2)–C(sp2) bond formation via C–H/C–F activation. Nat. Commun. 2015, 6, 7472–7479; (b) Wu, J.-Q.; Zhang, S.-S.; Gao, H.; Qi, Z.; Zhou, C.-J.; Ji, W.-W.; Liu, Y.; Chen, Y.; Li, Q.; Li, X.; Wang, H. Experimental and Theoretical Studies on Rhodium-Catalyzed Coupling of Benzamides with 2,2-Difluorovinyl Tosylate: Diverse Synthesis of Fluorinated Heterocycles. J. Am. Chem. Soc. 2017, 139, 3537–3545; (c) Thornbury, R. T.; Toste, F. D. Palladium-Catalyzed Defluorinative Coupling of 1-Aryl-2,2-Difluoroalkenes and Boronic Acids: Stereoselective Synthesis of Monofluorostilbenes. Angew. Chem. Int. Ed. 2016, 55, 11629–11632; (d) Lu, X.; Wang, Y.; Zhang, B.; Pi, J. J.; Wang, X. X.; Gong, T. J.; Xiao, B.; Fu, Y. Nickel-Catalyzed Defluorinative Reductive Cross-Coupling of gem-Difluoroalkenes with Unactivated Secondary and Tertiary Alkyl Halides. J. Am. Chem. Soc. 2017, 139, 12632–12637; (e) Dai, W.; Xiao, J.; Jin, G.; Wu, J.; Cao, S. Palladium- and Nickel-Catalyzed Kumada Cross-Coupling Reactions of gem-Difluoroalkenes and Monofluoroalkenes with Grignard Reagents. J. Org. Chem. 2014, 79, 10537–10546; (f) Xiong, Y.; Huang, T.; Ji, X.; Wu, J.; Cao, S. Nickel-catalyzed Suzuki–Miyaura Type Cross-coupling Reactions of (2,2-Difluorovinyl) Benzene Derivatives with Arylboronic acids. Org. Biomol. Chem. 2015, 13, 7389–7392; (g) Dai, W.; Shi, H.; Zhao, X.; Cao, S. Sterically Controlled Cu-Catalyzed or Transition-Metal-Free Cross-Coupling of gem-Difluoroalkenes with Tertiary, Secondary, and Primary Alkyl Grignard Reagents. Org. Lett. 2016, 18, 4284–4287; (h) Cai, S. H.; Ye, L.; Wang, D. X.; Wang, Y. Q.; Lai, L. J.; Zhu, C.; Feng, C.; Loh, T. P. Manganese-Catalyzed Synthesis of Monofluoroalkenes via C–H Activation and C–F Cleavage. Chem. Commun. 2017, 53, 8731–8734; (i) Yu, L.; Tang, M. L.; Si, C. M.; Meng, Z.; Liang, Y.; Han, J.; Sun, X. Zinc-Mediated Decarboxylative Alkylation of gem-Difluoroalkenes. Org. Lett. 2018, 20, 4579–4583; (j) Yang, L.; Ji, W. W.; Lin, E.; Li, J. L.; Fan, W. X.; Li, Q.; Wang, H. Synthesis of Alkylated Monofluoroalkenes via Fe-Catalyzed Defluorinative Cross-Coupling of Donor Alkenes with gem-Difluoroalkenes. Org. Lett. 2018, 20, 1924–1927; (k) Kondoh, A.; Koda, K.; Terada, M. Organocatalytic Nucleophilic Substitution Reaction of gem-Difluoroalkenes with Ketene Silyl Acetals. Org. Lett. 2019, 21, 2277–2280; (l) Zhang, J.; Dai, W.; Liu, Q.; Cao, S. Cu-Catalyzed Stereoselective Borylation of gem-Difluoroalkenes with B2pin2. Org. Lett. 2017, 19, 3283–3286; (m) Sakaguchi, H.; Uetake, Y.; Ohashi, M.; Niwa, T.; Ogoshi, S.; Hosoya, T. Copper-Catalyzed Regioselective Monodefluoroborylation of Polyfluoroalkenes en Route to Diverse Fluoroalkenes. J. Am. Chem. Soc. 2017, 139, 12855–12862; (n) Tan, D. H.; Lin, E.; Ji, W.-W.; Zeng, Y. F.; Fan, W.-X.; Li, Q.; Gao, H.; Wang, H. Copper-Catalyzed Stereoselective Defluorinative Borylation and Silylation of gem-Difluoroalkenes. Adv. Synth. Catal. 2018, 360, 1032–1037; (o) Kojima, R.; Kubota, K.; Ito, H. Stereodivergent Hydrodefluorination of gem-Difluoroalkenes: Selective Synthesis of (Z)- and (E)-Monofluoroalkenes. Chem. Commun. 2017, 53, 10688–10691; (p) Hu, J.; Han, X.; Yuan, Y.; Shi, Z. Stereoselective Synthesis of Z Fluoroalkenes through Copper-Catalyzed Hydrodefluorination of gem-Difluoroalkenes with Water Angew. Chem. Int. Ed. 2017, 56, 13342–13346; (q) Sakaguchi, H.; Ohashi, M.; Ogoshi, S. Fluorinated Vinylsilanes from the Copper-Catalyzed Defluorosilylation of Fluoroalkene Feedstocks. Angew. Chem. Int. Ed. 2018, 57, 328–332; (r) Li, J.; Lefebvre, Q.; Yang, H.; Zhao, Y.; Fu, H. Visible Light Photocatalytic Decarboxylative Monofluoroalkenylation of α-Amino Acids with gem-Difluoroalkenes. Chem. Commun. 2017, 53, 10299–10302; (s) Zhou, L.; Zhu, C.; Bi, P.; Feng, C. Ni-Catalyzed Migratory Fluoro-Alkenylation of Unactivated Alkyl Bromides with gem-Difluoroalkenes. Chem. Sci. 2019, 10, 1144–1149; (t) Xie, J.; Yu, J.; Rudolph, M.; Rominger, F.; Hashmi, A. S. K. Monofluoroalkenylation of Dimethylamino Compounds through Radical–Radical Cross-Coupling. Angew. Chem. Int. Ed. 2016, 55, 9416–9421.
- 6(a) van Steenis, J. H.; der Gen, A. V. Synthesis of Terminal Monofluoro-olefins. J. Chem. Soc., Perkin Trans. 2002, 1, 2117–2133;
10.1039/b106187a Google Scholar(b) Landelle, G.; Bergeron, M.; Turcotte-Savard, M. O.; Paquin, J. F. Synthetic Approaches to Monofluoroalkenes. Chem. Soc. Rev. 2011, 40, 2867–2908; (c) Drouin, M.; Hamel, J. D.; Paquin, J. F. Synthesis of Monofluoroalkenes: A Leap Forward. Synthesis 2018, 50, 881–955.
- 7 Dolbier, W. R.; Battiste, M. A. Structure, Synthesis, and Chemical Reactions of Fluorinated Cyclopropanes and Cyclopropenes. Chem. Rev. 2003, 103, 1071–1098.
- 8(a) Xu, J.; Ahmed, E. A.; Xiao, B.; Lu, Q. Q.; Wang, Y. L.; Yu, C. G.; Fu, Y. Pd-Catalyzed Regioselective Activation of gem-Difluorinated Cyclopropanes: A Highly Efficient Approach to 2-Fluorinated Allylic Scaffolds. Angew. Chem. Int. Ed. 2015, 54, 8231–8235; (b) Yang, W. C.; Chen, X.-B.; Song, K. L.; Wu, B.; Gan, W. E.; Zheng, Z. J.; Cao, J.; Xu, L. W. Pd-Catalyzed Enantioselective Tandem C–C Bond Activation/Cacchi Reaction between Cyclobutanones and o-Ethynylanilines. Org. Lett. 2021, 23, 1309–1314.
- 9(a) Wenz, J.; Rettenmeier, C. A.; Wadepohl, H.; Gade, L. H. Catalytic C–F Bond Activation of Geminal Difluorocyclopropanes by Nickel(I) Complexes via a Radical mechanism. Chem. Commun. 2016, 52, 202–205; (b) Song, X.; Xu, C.; Du, D.; Zhao, Z.; Zhu, D.; Wang, M. Ring-Opening Diarylation of Siloxydifluorocyclopropanes by Ag(I) Catalysis: Stereoselective Construction of 2-Fluoroallylic Scaffold. Org. Lett. 2017, 19, 6542–6545; (c) Ahmed, E. A. M. A.; Suliman, A. M. Y.; Gong, T. J.; Fu, Y. Palladium-Catalyzed Stereoselective Defluorination Arylation/Alkenylation/Alkylation of gem-Difluorinated Cyclopropanes. Org. Lett. 2019, 21, 5645–5649; (d) Ni, J.; Nishonov, B.; Pardaev, A.; Zhang, A. Palladium-Catalyzed Ring-Opening Coupling of gem-Difluorocyclopropanes for the Construction of 2-Fluoroallylic Sulfones. J. Org. Chem. 2019, 84, 13646–13654; (e) Ahmed, E. A. M. A.; Suliman, A. M. Y.; Gong, T. J.; Fu, Y. Access to Divergent Fluorinated Enynes and Arenes via Palladium-Catalyzed Ring-Opening Alkynylation of gem-Difluorinated Cyclopropanes. Org. Lett. 2020, 22, 1414–1419; (f) Liu, H.; Li, Y.; Wang, D. X.; Sun, M. M.; Feng, C. Visible-Light-Promoted Regioselective 1,3-Fluoroallylation of gem-Difluorocyclopropanes. Org. Lett. 2020, 22, 8681–8686; (g) Fu, Z.; Zhu, J.; Guo, S.; Lin, A. Palladium-Catalyzed Allylic Alkylation Dearomatization of β-Naphthols and Indoles with gem-Difluorinated Cyclopropanes. Chem. Commun. 2021, 57, 1262–1265; (h) Jiang, Z. T.; Huang, J.; Zeng, Y.; Hu, F.; Xia, Y. Rhodium Catalyzed Regioselective C−H Allylation of Simple Arenes via C−C Bond Activation of gem-Difluorinated Cyclopropanes. Angew. Chem. Int. Ed. 2021, 60, 10626–10631; (i) Lv, L.; Li, C. J. Pd-Catalyzed Defluorinative Alkylation of gem-Difluorocyclopropanes: Switching Regioselectivity via Simple Hydrazones. Angew. Chem. Int. Ed. 2021, 60, 13098–13104; (j) Lv, L.; Qian, H.; Ma, Y.; Huang, S.; Yan, X.; Li, Z. Ligand-Controlled Regioselective and Chemodivergent Defluorinative Functionalization of gem-Difluorocyclopropanes with Simple Ketones. Chem. Sci. 2021, 12, 15511–15518; (k) Suliman, A. M. Y.; Ahmed, E. A. M. A.; Gong, T. J.; Fu, Y. Cu/Pd-Catalyzed cis-Borylfluoroallylation of Alkynes for the Synthesis of Boryl-Substituted Monofluoroalkenes. Org. Lett. 2021, 23, 3259–3263; (l) Suliman, A. M. Y.; Ahmed, E. A. M. A.; Gong, T. J.; Fu, Y. Three-component Reaction of gem-Difluorinated Cyclopropanes with Alkenes and B2pin2 for the Synthesis of Monofluoroalkenes. Chem. Commun. 2021, 57, 6400–6403; (m) Xiong, B.; Chen, X.; Liu, J.; Zhang, X.; Xia, Y.; Lian, Z. Stereoselective gem-Difluorovinylation of gem-Difluorinated Cyclopropanes Enabled by Ni/Pd Cooperative Catalysis. ACS Catal. 2021, 11, 11960–11965; (n) Zhou, P. X.; Yang, X.; Wang, J.; Ge, C.; Feng, W.; Liang, Y. M.; Zhang, Y. Palladium-Catalyzed C–H Allylation of Electron-Deficient Polyfluoroarenes with gem-Difluorinated Cyclopropanes. Org. Lett. 2021, 23, 4920–4924; (o) Ai, Y.; Yang, H.; Duan, C.; Li, X.; Yu, S. Cobalt-Catalyzed Fluoroallyllation of Carbonyls via C–C Activation of gem-Difluorocyclopropanes. Org. Lett. 2022, 24, 5051–5055; (p) Lv, L.; Qian, H.; Crowell, A. B.; Chen, S.; Li, Z. Pd/NHC-Controlled Regiodivergent Defluorinative Allylation of gem-Difluorocyclopropanes with Allylboronates. ACS Catal. 2022, 12, 6495–6505; (q) Wu, L.; Wang, M.; Liang, Y.; Shi, Z. Ligand-Controlled Palladium-Catalyzed Regiodivergent Defluorinative Allylation of gem-Difluorocyclopropanes via σ-Bond Activation. Chin. J. Chem. 2022, 40, 2345–2355; (r) Wu, X.; Zeng, Y.; Jiang, Z.-T.; Zhu, Y.; Xie, L.; Xia, Y. Lewis Acid-Catalyzed Ring-Opening Cross-Coupling Reaction of gem-Difluorinated Cyclopropanes Enabled by C–F Bond Activation. Org. Lett. 2022, 24, 8429–8434; (s) Yuan, W.; Li, X.; Qi, Z.; Li, X. Palladium-Catalyzed Synthesis of Functionalized Indoles by Acylation/Allylation of 2-Alkynylanilines with Three-Membered Rings. Org. Lett. 2022, 24, 2093–2098; (t) Zeng, Y.; Gao, H.; Zhu, Y.; Jiang, Z. T.; Lu, G.; Xia, Y. Site-Divergent Alkenyl C–H Fluoroallylation of Olefins Enabled by Tunable Rhodium Catalysis. ACS Catal. 2022, 12, 8857–8867; (u) Zeng, Y.; Yang, H.; Du, J.; Huang, Q.; Huang, G.; Xia, Y. Rh-Catalyzed Regio-Switchable Cross-Coupling of gem-Difluorinated Cyclopropanes with Allylboronates to Structurally Diverse Fluorinated Dienes. Chem. Sci. 2022, 13, 12419–12425; (v) Qian, H.; Nguyen, H. D.; Lv, L.; Chen, S.; Li, Z. Chemo-, Stereo- and Regioselective Fluoroallylation/Annulation of Hydrazones with gem-Difluorocyclopropanes via Tunable Palladium/NHC Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202303271; (w) Li, D.; Shen, C.; Si, Z.; Liu, L. Palladium-Catalyzed Fluorinative Bifunctionalization of Aziridines and Azetidines with gem-Difluorocyclopropanes. Angew. Chem. Int. Ed. 2023, 62, e202310283.
- 10(a) Song, X.; Xu, C.; Wang, M. Transformations Based on Ring- Opening of gem-Difluorocyclopropanes. Tetrahedron Lett. 2017, 58, 1806–1816; (b) Lv, L.; Qian, H.; Li, Z. Catalytic Diversification of gem-Difluorocyclopropanes: Recent Advances and Challenges. ChemCatChem 2022, 14, e202200890; (c) Zhu, Y.; Zeng, Y.; Jiang, Z. T.; Xia, Y. Recent Advances in Transition-Metal-Catalyzed Cross-Coupling Reactions of gem-Difluorinated Cyclopropanes. Synlett 2022, 34, 1–13.
- 11(a) Tasker, S. Z.; Standley, E. A.; Jamison, T. F. Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309; (b) Diccianni, J.; Lin, Q.; Diao, T. Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization. Acc. Chem. Res. 2020, 53, 906–919.
- 12(a) Freixa, Z.; van Leeuwen, P. W. N. M. Bite Angle Effects in Diphosphine Metal Catalysts: Steric or Electronic. Dalton Trans. 2003, 1890–1901; (b) Birkholz, M.-N.; Freixa, Z.; van Leeuwen, P. W. N. M. Bite Angle Effects of Diphosphines in C–C and C–X Bond Forming Cross-Coupling Reactions. Chem. Soc. Rev. 2009, 38, 1099–1118.
- 13(a) Lennox A. J. J.; Lloyd-Jones, G. C. The Slow-Release Strategy in Suzuki–Miyaura Coupling. Isr. J. Chem. 2010, 50, 664–674; (b) Cox, P. A.; Reid, M.; Leach, A. G.; Campbell, A. G.; King, E. J.; Lloyd-Jones, G. C. Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From Concerted Proton Transfer to Liberation of a Transient Aryl Anion. J. Am. Chem. Soc. 2017, 139, 13156–1316




