Enantioselective Synthesis of Benzimidazole Atropisomers Featuring a N-N Axis†
Fang-Bei Ge
College of Chemistry and Chemical Engineering, Qingdao University, Qingdao, Shandong, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorQi-Kun Yin
School of Pharmacy, Key Laboratory of Molecular Pharmacology and Drug Evaluation, Ministry of Education, Yantai University, Yantai, Shandong, 264005 China
These authors contributed equally to this work.
Search for more papers by this authorChuan-Jun Lu
College of Chemistry and Chemical Engineering, Qingdao University, Qingdao, Shandong, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorXuan Xuan
School of Pharmacy, Key Laboratory of Molecular Pharmacology and Drug Evaluation, Ministry of Education, Yantai University, Yantai, Shandong, 264005 China
Search for more papers by this authorJia Feng
College of Chemistry and Chemical Engineering, Qingdao University, Qingdao, Shandong, 266071 China
Search for more papers by this authorCorresponding Author
Ren-Rong Liu
College of Chemistry and Chemical Engineering, Qingdao University, Qingdao, Shandong, 266071 China
E-mail: [email protected]Search for more papers by this authorFang-Bei Ge
College of Chemistry and Chemical Engineering, Qingdao University, Qingdao, Shandong, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorQi-Kun Yin
School of Pharmacy, Key Laboratory of Molecular Pharmacology and Drug Evaluation, Ministry of Education, Yantai University, Yantai, Shandong, 264005 China
These authors contributed equally to this work.
Search for more papers by this authorChuan-Jun Lu
College of Chemistry and Chemical Engineering, Qingdao University, Qingdao, Shandong, 266071 China
These authors contributed equally to this work.
Search for more papers by this authorXuan Xuan
School of Pharmacy, Key Laboratory of Molecular Pharmacology and Drug Evaluation, Ministry of Education, Yantai University, Yantai, Shandong, 264005 China
Search for more papers by this authorJia Feng
College of Chemistry and Chemical Engineering, Qingdao University, Qingdao, Shandong, 266071 China
Search for more papers by this authorCorresponding Author
Ren-Rong Liu
College of Chemistry and Chemical Engineering, Qingdao University, Qingdao, Shandong, 266071 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
Atroposelective synthesis of N-N atropisomers is an emerging area but remains underexplored; in particular, the synthesis of N-N benzimidazole atropisomers is still unprecedented. Herein, the first enantioselective synthesis of N-N benzimidazole atropisomers via the palladium-catalyzed de novo construction of benzimidazole skeleton is presented. With readily available palladium catalyst and biphosphine ligand, a broad range of nonbiaryl benzimidazole and indole-benzimidazole atropisomers were conveniently accessed in high yields and with excellent enantioselectivities. Significantly, these N-N benzimidazole atropisomers showed great antitumor activity and selectivity to breast cancer MCF-7 cells. The simple catalytic system, broad substrate scope, high enantioselectivity, and good bioactivity make this approach highly attractive.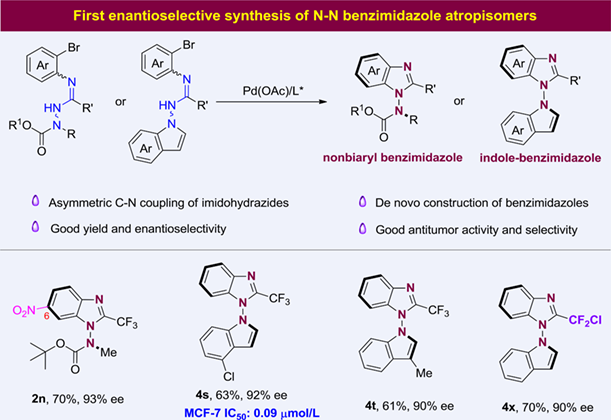
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300600-sup-0001-supinfo.pdfPDF document, 26.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Bringmann, G.; Tasler, S.; Endress, H.; Kraus, J.; Messer, K.; Wohlfarth, M.; Lobin, W. Murrastifoline-F: First Total Synthesis, Atropo-Enantiomer Resolution, and Stereoanalysis of an Axially Chiral N,C-Coupled Biaryl Alkaloid. J. Am. Chem. Soc. 2001, 123, 2703−2711; (b) Hughes, C. C.; Prieto-Davo, A.; Jensen, P. R.; Fenical, W. The Marinopyrroles, Antibiotics of an Unprecedented Structure Class from a Marine Streptomyces sp. Org. Lett. 2008, 10, 629−631; (c) Toenjes, S. T.; Gustafson, J. L. Atropisomerism in medicinal chemistry: challenges and opportunities. Future Med. Chem. 2018, 10, 409; (d) LaPlante, S. R.; Fader, L. R.; Fandrick, K. R.; Fandrick, D. R.; Hucke, O.; Kemper, R.; Miller, S. P. F.; Edwards, P. J. J. Assessing Atropisomer Axial Chirality in Drug Discovery and Development. Med. Chem. 2011, 54, 7005−7022; (e) Kriegelstein, M.; Profous, D.; Lyčka, A.; Travnícek, Z.; Přibylka, A.; Volná, T.; Benická, S.; Cankař, P. Axially Chiral Trifluoromethylbenzimidazolylbenzoic Acid: A Chiral Derivatizing Agent for α-Chiral Primary Amines and Secondary Alcohols to Determine the Absolute Configuration. J. Org. Chem. 2019, 84, 11911−11921; (f) Perreault, S.; Chandrasekhar, J.; Patel, L. Atropisomerism in Drug Discovery: A Medicinal Chemistry Perspective Inspired by Atropisomeric Class I PI3K Inhibitors. Acc. Chem. Res. 2022, 55, 2581−2593; (g) Feng, J.; Lu, C.-J.; Liu, R.-R. Catalytic Asymmetric Synthesis of Atropisomers Featuring an Aza Axis. Acc. Chem. Res. 2023, 56, 2537–2554; (h) Zhang, P.; Feng, J.; Liu, R.-R. Enantioselective Synthesis of Axially Chiral Benzimidazoles Bearing a C-N axis via Pd-Catalyzed Buchwald–Hartwig Amination. Synlett 2022, 33, 1589–1595; (i) Cheng, J. K.; Xiang, S. H.; Li, S.; Ye, L.; Tan, B. Recent Advances in Catalytic Asymmetric Construction of Atropisomers. Chem. Rev. 2021, 121, 4805−4902; (j) Colobert.; Shi, B. F. C-N atropopure compounds: New directions. Chem. Catal. 2021, 1, 483−485; (k) Zhang, H.-H.; Shi, F.; Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications. Acc. Chem. Res. 2022, 55, 2562−2580; (l) Fan, X. Z.; Zhang, X.; Li, C. Y.; Gu, Z. H. Enantioselective Atropisomeric Anilides Synthesis via Cu-Catalyzed Intramolecular Adjacent C-N Coupling. ACS Catal. 2019, 9, 2286−2291.
- 2(a) Kwon, Y.; Li, J.; Reid, J. P.; Crawford, J. M.; Jacob, R.; Sigman, M. S.; Toste, F. D.; Miller, S. J. Disparate Catalytic Scaffolds for Atroposelective Cyclodehydration. J. Am. Chem. Soc. 2019, 141, 6698−6705; (b) Man, N.; Lou, Z.; Li, Y.; Yang, H.; Zhao, Y.; Fu, H. Organocatalytic Atroposelective Construction of Axially Chiral N-Aryl Benzimidazoles Involving Carbon-Carbon Bond Cleavage. Org. Lett. 2020, 22, 6382−6387.
- 3 An, Q. J.; Xia, W.; Ding, W. Y.; Liu, H. H.; Xiang, S. H.; Wang, Y. B.; Zhong, G.; Tan, B. Nitrosobenzene-Enabled Chiral Phosphoric Acid Catalyzed Enantioselective Construction of Atropisomeric N-Arylbenzimidazoles. Angew. Chem. Int. Ed. 2021, 60, 24888−24893.
- 4 Zhang, P.; Wang, X. M.; Xu, Q.; Guo, C. Q.; Wang, P.; Lu, C. J.; Liu, R. R. Enantioselective Synthesis of Atropisomeric Biaryls by Pd-Catalyzed Asymmetric Buchwald-Hartwig Amination. Angew. Chem. Int. Ed. 2021, 60, 21718−21722.
- 5(a) Mei, G. Y.; Koay, W. L.; Guan, C. Y.; Lu, Y. Atropisomers beyond the C-C axial chirality: Advances in catalytic asymmetric synthesis. Chem 2023, 8, 1855−1893; (b) Song, T. Y.; Li, R.; Huang, L.; Jia, S. K.; Mei, G. Y. Catalytic Asymmetric Synthesis of N-N Atropisomers. Chin. J. Org. Chem. 2023, 43, 1977−1990; (c) Centonze, G.; Portolani, C.; Righi, P.; Bencivenni, G. Enantioselective Strategies for The Synthesisof N-N Atropisomers. Angew. Chem. Int. Ed. 2023, 62, 202303966.
- 6 Chang, C.; Adams, R. Stereochemistry of N,N’-Dipyrryls. Resolution of N,N’,2,5,2’,5’-Tetramethyl-3,3’-dicarboxydipyrryl. XVI. J. Am. Chem. Soc. 1931, 53, 2353−2357.
- 7 Wang, X. M.; Zhang, P.; Xu, Q.; Guo, C. Q.; Zhang, D. B.; Lu, C. J.; Liu, R. R. Enantioselective Synthesis of Nitrogen-Nitrogen Biaryl Atropisomers via Copper-Catalyzed Friedel-Crafts Alkylation Reaction. J. Am. Chem. Soc. 2021, 143, 15005−15010.
- 8 Xu, Q.; Zhang, H.; Ge, F. B.; Wang, X. M.; Zhang, P.; Lu, C. J.; Liu, R. R. Cu(I)-Catalyzed Asymmetric Arylation of Pyrroles with Diaryliodonium Salts toward the Synthesis of N-N Atropisomers. Org. Lett. 2022, 24, 3138−3143.
- 9 Mei, G. J.; Wong, J. J.; Zheng, W.; Nangia, A. A.; Houk, K. N.; Lu, Y. Rational design and atroposelective synthesis of N-N axially chiral compounds. Chem 2021, 7, 2743−2757.
- 10(a) Lin, W.; Zhao, Q.; Li, Y.; Pan, M.; Yang, C.; Yang, G.; Li, X. Asymmetric synthesis of N-N axially chiral compounds via organocatalytic atroposelective N-acylation. Chem. Sci. 2022, 13, 141−148; (b) Pan, M.; Shao, Y. B.; Zhao, Q.; Li, X. Asymmetric Synthesis of N-N Axially Chiral Compounds by Phase-Transfer-Catalyzed Alkylations. Org. Lett. 2022, 24, 374−378; (c) Portolani, C.; Centonze, G.; Luciani, S.; Pellegrini, A.; Righi, P.; Mazzanti, A.; Ciogli, A.; Sorato, A.; Righi, P.; Bencivenni, G. Synthesis of Atropisomeric Hydrazides by One-Pot Sequential Enantio- and Diastereoselective Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202209895.
- 11(a) Chen, K. W.; Chen, Z. H.; Yang, S.; Wu, S. F.; Zhang, Y. C.; Shi, F. Organocatalytic Atroposelective Synthesis of N−N Axially Chiral Indoles and Pyrroles by De Novo Ring Formation. Angew. Chem. Int. Ed. 2022, 61, e202116829; (b) Gao, Y.; Wang, L. Y.; Zhang, T.; Yang, B. M.; Zhao, Y. Atroposelective Synthesis of 1,1’-Bipyrroles Bearing a Chiral N−N Axis: Chiral Phosphoric Acid Catalysis with Lewis Acid Induced Enantiodivergence. Angew. Chem. Int. Ed. 2022, 61, e202200371.
- 12 Pu, L. Y.; Zhang, Y. J.; Liu, W.; Teng, F. Chiral phosphoric acid-catalyzed dual-ring formation for enantioselective construction of N-N axially chiral 3,3’-bisquinazolinones. Chem. Commun. 2022, 58, 13131−13134.
- 13(a) Chen, Z. H.; Li, T. Z.; Wang, N. Y.; Ma, X. F.; Ni, S. F.; Zhang, Y. C.; Shi, F. Organocatalytic Enantioselective Synthesis of Axially Chiral N,N’-Bisindoles. Angew. Chem. Int. Ed. 2023, 62, e202300419; (b) Wang, L. Y.; Miao, J.; Zhao, Y.; Yang, B. M. Chiral Acid-Catalyzed Atroposelective Indolization Enables Access to 1,1’-Indole-Pyrroles and Bisindoles Bearing a Chiral N–N Axis. Org. Lett. 2023, 25, 1553−1557.
- 14 Hutskalova, V.; Sparr, C. Control over Stereogenic N–N Axes by Pd-Catalyzed 5-endo-Hydroaminocyclizations. Synthesis 2023, 55, 1770−1782.
- 15 Zhang, P.; Xu, Q.; Wang, X. M.; Feng, J.; Lu, C. J.; Li, Y.; Liu, R. R. Enantioselective Synthesis of N−N Bisindole Atropisomers. Angew. Chem. Int. Ed. 2022, 61, e202212101.
- 16(a) Yao, W.; Lu, C. J.; Zhan, L. W.; Wu, Y.; Feng, J.; Liu, R. R. Enantioselective Synthesis of N-N Atropisomers by Palladium-Catalyzed C−H Functionalization of Pyrroles. Angew. Chem. Int. Ed. 2023, 62, e202218871; (b) Yin, S. L.; Zhou, Q.; Liu, C. X.; Gu, Q.; You, S. L. Enantioselective Synthesis of N−N Biaryl Atropisomers through Iridium(I)-Catalyzed C−H Alkylation with Acrylates. Angew. Chem. Int. Ed. 2023, 62, e202305067; (c) Wang, Y.; Zhu, X.; Pan, D.; Wan, F.; Mi, R.; Huang, G.; Li, X. Rhodium-catalyzed enantioselective and diastereodivergent access to diaxially chiral heterocycles. Nat. Commun. 2023, 14, 4661; (d) Zhu, X.; Wu, H.; Wang, Y.; Huang, G.; Wang, F.; Li, X. Rhodium-catalyzed annulative approach to N-N axially chiral biaryls via C-H activation and dynamic kinetic transformation. Chem. Sci. 2023, 14, 8564−8569; (e) Wang, C.; Sun, J. Atroposelective Synthesis of N-N Axially Chiral Bipyrroles via Rhodium-Catalyzed C-H Insertion Reaction. Org. Lett. 2023, 25, 4808−4812; (f) Li, T.; Shi, L.; Wang, X.; Yang, C.; Yang, D.; Song, M.-P.; Niu, J.-L. Cobalt-catalyzed atroposelective C-H activation/annulation to access N-N axially chiral frameworks. Nat. Commun. 2023, 14, 5271.
- 17For atroposelective synthesis of indole and pyrrole atropisomers, see: (a) Da, B. C.; Xiang, S. H.; Li, S. Y.; Tan, B. Chiral Phosphoric Acid Catalyzed Asymmetric Synthesis of Axially Chiral Compounds. Chin. J. Chem. 2021, 39, 1787−1796;
(b) Wang, J. L.; Sun, M.; Yu, X. Y.; Zhang, Y. C.; Tan, W.; Shi, F. Atroposelective Construction of Axially Chiral Alkene-Indole Scaffolds via Catalytic Enantioselective Addition Reaction of 3-Alkynyl-2-indolylmethanols. Chin. J. Chem. 2021, 39, 2163−2171;
(c) Sheng, F. T.; Yang, S.; Wu, S. H.; Zhang, Y. C.; Shi, F. Catalytic Asymmetric Synthesis of Axially Chiral 3,3'-Bisindoles by Direct Coupling of Indole Rings. Chin. J. Chem. 2022, 40, 2151−2160;
(d) Huang, Q. Q.; Wu, S. F.; Yang, S.; Wang, X.; Zhong, Z.; Zhang, Y. C.; Shi, F. Design and catalytic atroposelective synthesis of axially chiral isochromenone-indoles. Sci. China Chem. 2022, 65, 1929−1937;
(e) Wang, L.; Yuan, W. K.; Wang, Z. K.; Luo, J.; Zhou, T.; Shi, B. F. Synthesis of C-N Axial Chirality N-Arylindoles via Pd(II)-Catalyzed Free Amine-Directed Atroposelective C-H Olefination. Chin. J. Chem. 2023, 41, 2788-2792;
(f) Wu, P.; Yu, L.; Gao, C. H.; Chen, Q.; Deng, S.; Jiao, Y. C.; Tan, W.; Shi, F. Design and synthesis of axially chiral aryl-pyrroloindoles via the strategy of organocatalytic asymmetric (2 + 3) cyclization. Fundam. Res. 2023, 3, 237−248;
(g) Liu, Y. H.; Chen, Y. H.; Cheng, J. K.; Xiang, S. H.; Tan, B. Enantioselective synthesis of 3-arylindole atropisomers via organocatalytic indolization of iminoquinones. Chem. Synth. 2023, 3, 11;
(h) Zhang, H.-H.; Li, T.-Z.; Liu, S.-J.; Shi, F. Catalytic Asymmetric Synthesis of Atropisomers Bearing Multipl Chiral Elements: An Emerging Field. Angew. Chem. Int. Ed. 2023, 62, DOI: https://doi.org/10.1002/anie.202311053.
10.1002/anie.202311053 Google Scholar
- 18 Lu, C. J.; Xu, Q.; Feng, J.; Liu, R. R. The Asymmetric Buchwald-Hartwig Amination Reaction. Angew. Chem. Int. Ed. 2023, 62, e202216863.
- 19The cytotoxicity of representative benzimidazoles (4t and 4s) in different chiralities was also revaluated using the MTT assay. The IC50 values of (S)-4s and (S)-4t were determined as 18.2 and 23.6 μmol/L to MCF-7 cells, suggesting that the chirality change could significantly decrease the cytotoxicity of 4t and 4s.
| Cell lines | IC50 values of compound 4/(μmol·L–1) | |||
|---|---|---|---|---|
| 4t | 4s | (S)-4s | (S)-4t | |
| MCF-7 | 2.1 | 0.09 | 18.2 | 23.6 |
| B16 | > 50 | > 50 | > 50 | > 50 |
| A549 | > 50 | > 50 | > 50 | > 50 |
| HepG2 | > 50 | > 50 | > 50 | > 50 |
| HL7702 | > 50 | > 50 | > 50 | > 50 |




