Supported Atomically Dispersed Pd Catalyzed Direct Alkoxylation and Allylic Alkylation†
Corresponding Author
Ruixuan Qin
New Cornerstone Science Laboratory, State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and National & Local Joint Engineering Research Center of Preparation Technology of Nanomaterials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, Fujian, 361102 China
Fujian Key Laboratory of Rare-earth Functional Materials, Fujian Shanhai Collaborative Innovation Center of Rare-earth Functional Materials, Longyan, Fujian, 366300 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZiwen Chen
New Cornerstone Science Laboratory, State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and National & Local Joint Engineering Research Center of Preparation Technology of Nanomaterials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
School of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210 China
Search for more papers by this authorQingyuan Wu
New Cornerstone Science Laboratory, State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and National & Local Joint Engineering Research Center of Preparation Technology of Nanomaterials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, Fujian, 361102 China
Search for more papers by this authorNanfeng Zheng
New Cornerstone Science Laboratory, State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and National & Local Joint Engineering Research Center of Preparation Technology of Nanomaterials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, Fujian, 361102 China
Fujian Key Laboratory of Rare-earth Functional Materials, Fujian Shanhai Collaborative Innovation Center of Rare-earth Functional Materials, Longyan, Fujian, 366300 China
Search for more papers by this authorCorresponding Author
Pengxin Liu
School of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ruixuan Qin
New Cornerstone Science Laboratory, State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and National & Local Joint Engineering Research Center of Preparation Technology of Nanomaterials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, Fujian, 361102 China
Fujian Key Laboratory of Rare-earth Functional Materials, Fujian Shanhai Collaborative Innovation Center of Rare-earth Functional Materials, Longyan, Fujian, 366300 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZiwen Chen
New Cornerstone Science Laboratory, State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and National & Local Joint Engineering Research Center of Preparation Technology of Nanomaterials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
School of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210 China
Search for more papers by this authorQingyuan Wu
New Cornerstone Science Laboratory, State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and National & Local Joint Engineering Research Center of Preparation Technology of Nanomaterials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, Fujian, 361102 China
Search for more papers by this authorNanfeng Zheng
New Cornerstone Science Laboratory, State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and National & Local Joint Engineering Research Center of Preparation Technology of Nanomaterials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian, 361005 China
Innovation Laboratory for Sciences and Technologies of Energy Materials of Fujian Province (IKKEM), Xiamen, Fujian, 361102 China
Fujian Key Laboratory of Rare-earth Functional Materials, Fujian Shanhai Collaborative Innovation Center of Rare-earth Functional Materials, Longyan, Fujian, 366300 China
Search for more papers by this authorCorresponding Author
Pengxin Liu
School of Physical Science and Technology, ShanghaiTech University, Shanghai, 201210 China
E-mail: [email protected]; [email protected]Search for more papers by this authorDedicated to the Special Issue of Single-Atom Catalysis.
Comprehensive Summary
A new approach to allylic alkylation is realized using an atomically dispersed palladium catalyst (Pd1/TiO2-EG). Unlike conventional methods that require derivation of substrates and utilization of additives, this method allows for direct allylic alkylation from allylic alcohols, producing H2O as the sole by-product. The catalyst's high efficiency is attributed to the local hydrogen bonding at the organic-inorganic interface (Pd-EG interface), facilitating hydroxyl group activation for η3 π-allyl complex formation. The system demonstrates successful direct C—O and C—C coupling reactions with high selectivity, requiring no additives. This study highlights the potential of supported atomically dispersed catalysts for greener and more efficient catalysis, meanwhile, offers unique insights into the distinct behavior of atomically dispersed catalysts in comparison to homogeneous or nanoparticle-based catalysts.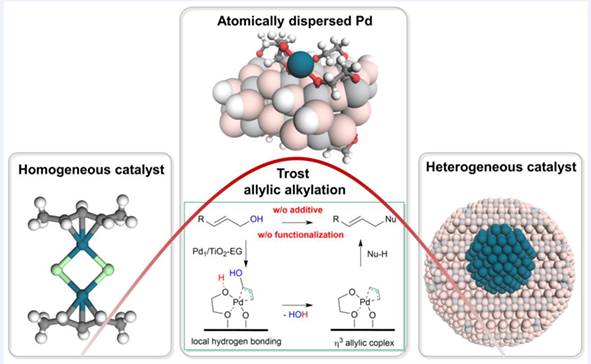
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300516-sup-0001-supinfo.pdfPDF document, 1.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Trost, B. M.; Kaneko, T.; Andersen, N. G.; Tappertzhofen, C.; Fahr, B. Total synthesis of aeruginosin 98B. J. Am. Chem. Soc. 2012, 134, 18944–18947.
- 2 Trost, B. M.; Crawley, M. L. Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem. Rev. 2003, 103, 2921–2944.
- 3 Sundararaju, B.; Achard, M.; Bruneau, C. Transition metal catalyzed nucleophilic allylic substitution: activation of allylic alcohols via pi-allylic species. Chem. Soc. Rev. 2012, 41, 4467–4483.
- 4 Kimura, M.; Horino, Y.; Mukai, R.; Tanaka, S.; Tamaru, Y. Strikingly simple direct alpha-allylation of aldehydes with allyl alcohols: remarkable advance in the Tsuji-Trost reaction. J. Am. Chem. Soc. 2001, 123, 10401–10402.
- 5 Lysenko, I. L.; Kim, K.; Lee, H. G.; Cha, J. K. Low-valent titanium- mediated stereoselective alkylation of allylic alcohols. J. Am. Chem. Soc. 2008, 130, 15997–6002.
- 6 Jiang, G.; List, B. Palladium/Brønsted Acid-Catalyzed α-Allylation of Aldehydes with Allylic Alcohols. Adv. Synth. Catal. 2011, 353, 1667–1670.
- 7 Ohshima, T.; Miyamoto, Y.; Ipposhi, J.; Nakahara, Y.; Utsunomiya, M.; Mashima, K. Platinum-catalyzed direct amination of allylic alcohols under mild conditions: ligand and microwave effects, substrate scope, and mechanistic study. J. Am. Chem. Soc. 2009, 131, 14317–14328.
- 8 Piechaczyk, O.; Thoumazet, C.; Jean, Y.; le Floch, P. DFT study on the palladium-catalyzed allylation of primary amines by allylic alcohol. J. Am. Chem. Soc. 2006, 128, 14306–14317.
- 9 Trost, B. M.; Zhang, T.; Sieber, J. D. Catalytic asymmetric allylic alkylation employing heteroatom nucleophiles: a powerful method for C–X bond formation. Chem. Sci. 2010, 1, 427–440.
- 10
Song, C. E.; Yang, J. W.; Roh, E. J.; Lee, S. G.; Ahn, J. H.; Han, H. Heterogeneous Pd-catalyzed asymmetric allylic substitution using resin-supported trost-type bisphosphane ligands. Angew. Chem. Int. Ed. 2002, 41, 3852–3854.
10.1002/1521-3773(20021018)41:20<3852::AID-ANIE3852>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 11
Mitsudome, T.; Nose, K.; Mori, K.; Mizugaki, T.; Ebitani, K.; Jitsukawa, K.; Kaneda, K. Montmorillonite-Entrapped Sub-nanoordered Pd Clusters as a Heterogeneous Catalyst for Allylic Substitution Reactions. Angew. Chem. Int. Ed. 2007, 119, 3352–3354.
10.1002/ange.200604644 Google Scholar
- 12 Bergbreiter, D. E.;Chen, B. Allylic Substitution Using Heterogeneous Palladium Catalysts. J. Chem. Soc., Chem. Commun. 1983, 0, 1238–1239.
- 13 Hemelaere, R.; Desroches, J.; Paquin, J. F. Introduction of the 4,4,4-trifluorobut-2-ene chain exploiting a regioselective Tsuji-Trost reaction catalyzed by palladium nanoparticles. Org. Lett. 2015, 17, 1770–1773.
- 14 Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397.
- 15 Chen, F.; Jiang, X.; Zhang, L.; Lang, R.; Qiao, B. Single-atom catalysis: Bridging the homo- and heterogeneous catalysis. Chin. J. Catal. 2018, 39, 893–898.
- 16 Fu, N.; Liang, X.; Li, Z.; Chen, W.; Wang, Y.; Zheng, L.; Zhang, Q.; Chen, C.; Wang, D.; Peng, Q.; Gu, L.; Li, Y. Fabricating Pd isolated single atom sites on C3N4/rGO for heterogenization of homogeneous catalysis. Nano Res. 2020, 13, 947–951.
- 17 Saptal, V. B.; Ruta, V.; Bajada, M. A.; Vilé, G. Single-Atom Catalysis in Organic Synthesis. Angew. Chem. Int. Ed. 2023, 62, e202219306.
- 18 Hu, H.; Xi, J. Single-atom catalysis for organic reactions. Chin. Chem. Lett. 2023, 34, 107959.
- 19 Wu, F.; Liu, P. Surface Organometallic Chemistry for Single-site Catalysis and Single-atom Catalysis. Chem. Res. Chin. Univ. 2022, 38, 1139–1145.
- 20 Jing, W.; Shen, H.; Qin, R.; Wu, Q.; Liu, K.; Zheng, N. Surface and Interface Coordination Chemistry Learned from Model Heterogeneous Metal Nanocatalysts: From Atomically Dispersed Catalysts to Atomically Precise Clusters. Chem. Rev. 2023, 123, 5948–6002.
- 21 Qin, R.; Liu, K.; Wu, Q.; Zheng, N. Surface Coordination Chemistry of Atomically Dispersed Metal Catalysts. Chem. Rev. 2020, 120, 11810–11899.
- 22 Trost, B. M.; Fullerton, T. J. New synthetic reactions. Allylic alkylation. J. Am. Chem. Soc. 1973, 95, 292–294.
- 23 Trost, B. M.; Van Vranken, D. L. Asymmetric Transition Metal-Catalyzed Allylic Alkylations. Chem. Rev. 1996, 96, 395–422.
- 24 Trost, B. M. Asymmetric allylic alkylation, an enabling methodology. J. Org. Chem. 2004, 69, 5813–5837.
- 25 Zhao, Q.; Zhu, Y.; Sun, Z.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Combining palladium complex and organic amine on graphene oxide for promoted Tsuji–Trost allylation. J. Mater. Chem. A 2015, 3, 2609–2616.
- 26 Uozumi, Y.; Shibatomi, K. Catalytic asymmetric allylic alkylation in water with a recyclable amphiphilic resin-supported P,N-chelating palladium complex. J. Am. Chem. Soc. 2001, 123, 2919–2920.
- 27 Dickschat, A. T.; Behrends, F.; Surmiak, S.; Weiss, M.; Eckert, H.; Studer, A. Bifunctional mesoporous silica nanoparticles as cooperative catalysts for the Tsuji-Trost reaction–tuning the reactivity of silica nanoparticles. Chem. Commun. 2013, 49, 2195–2197.
- 28 Liu, P.; Zhao, Y.; Qin, R.; Mo, S.; Chen, G.; Gu, L.; Chevrier, D. M.; Zhang, P.; Guo, Q.; Zang, D.; Wu, B.; Fu, G.; Zheng, N. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 2016, 352, 797–801.
- 29 Liu, P.; Zhao, Y.; Qin, R.; Gu, L.; Zhang, P.; Fu, G.; Zheng, N. A vicinal effect for promoting catalysis of Pd1/TiO2: supports of atomically dispersed catalysts play more roles than simply serving as ligands. Sci. Bull. 2018, 63, 675–682.
- 30 Walker, W. K.; Kay, B. M.; Michaelis, S. A.; Anderson, D. L.; Smith, S. J.; Ess, D. H.; Michaelis, D. J. Origin of fast catalysis in allylic amination reactions catalyzed by Pd-Ti heterobimetallic complexes. J. Am. Chem. Soc. 2015, 137, 7371–7378.
- 31 Ghebreghiorgis, T.; Kirk, B. H.; Aponick, A.; Ess, D. H. Multiple mechanisms in Pd(II)-catalyzed SN2' reactions of allylic alcohols. J. Org. Chem. 2013, 78, 7664–7673.
- 32 Gumrukcu, Y.; de Bruin, B.; Reek, J. A Mechanistic Study of Direct Activation of Allylic Alcohols in Palladium Catalyzed Amination Reactions. Catalysts 2015, 5, 349–365.
- 33 Gumrukcu, Y.; de Bruin, B.; Reek, J. N. Hydrogen-bond-assisted activation of allylic alcohols for palladium-catalyzed coupling reactions. ChemSusChem 2014, 7, 890–896.
- 34 Huo, X.; Quan, M.; Yang, G.; Zhao, X.; Liu, D.; Liu, Y.; Zhang, W. Hydrogen-bond-activated palladium-catalyzed allylic alkylation via allylic alkyl ethers: challenging leaving groups. Org. Lett. 2014, 16, 1570–1573.
- 35 Huo, X.; Yang, G.; Liu, D.; Liu, Y.; Gridnev, I. D.; Zhang, W. Palladium-catalyzed allylic alkylation of simple ketones with allylic alcohols and its mechanistic study. Angew. Chem. Int. Ed. 2014, 53, 6776–6780.
- 36 Chen, F.; Li, T.; Pan, X.; Guo, Y.; Han, B.; Liu, F.; Qiao, B.; Wang, A.; Zhang, T. Pd1/CeO2 single-atom catalyst for alkoxycarbonylation of aryl iodides. Sci. China Mater. 2020, 63, 959–964.
- 37 Zhang, X.; Sun, Z.; Wang, B.; Tang, Y.; Nguyen, L.; Li, Y.; Tao, F. F. C-C Coupling on Single-Atom-Based Heterogeneous Catalyst. J. Am. Chem. Soc. 2018, 140, 954–962.
- 38 Lang, R.; Li, T.; Matsumura, D.; Miao, S.; Ren, Y.; Cui, Y. T.; Tan, Y.; Qiao, B.; Li, L.; Wang, A.; Wang, X.; Zhang, T. Hydroformylation of Olefins by a Rhodium Single-Atom Catalyst with Activity Comparable to RhCl(PPh3)3. Angew. Chem. Int. Ed. 2016, 55, 16054–16058.
- 39 Wang, L.; Zhang, W.; Wang, S.; Gao, Z.; Luo, Z.; Wang, X.; Zeng, R.; Li, A.; Li, H.; Wang, M.; Zheng, X.; Zhu, J.; Zhang, W.; Ma, C.; Si, R.; Zeng, J. Atomic-level insights in optimizing reaction paths for hydroformylation reaction over Rh/CoO single-atom catalyst. Nat. Commun. 2016, 7, 14036.
- 40 Zhang, L.; Wang, A.; Miller, J. T.; Liu, X.; Yang, X.; Wang, W.; Li, L.; Huang, Y.; Mou, C.-Y.; Zhang, T. Efficient and Durable Au Alloyed Pd Single-Atom Catalyst for the Ullmann Reaction of Aryl Chlorides in Water. ACS Catal. 2014, 4, 1546–1553.
- 41 Ren, S.; Ye, B.; Li, S.; Pang, L.; Pan, Y.; Tang, H. Well-defined coordination environment breaks the bottleneck of organic synthesis: Single-atom palladium catalyzed hydrosilylation of internal alkynes. Nano Res. 2022, 15, 1500–1508.
- 42 Liu, K.; Badamdorj, B.; Yang, F.; Janik, M. J.; Antonietti, M. Accelerated Anti-Markovnikov Alkene Hydrosilylation with Humic- Acid-Supported Electron-Deficient Platinum Single Atoms. Angew. Chem. Int. Ed. 2021, 60, 24220–24226.
- 43 Zhao, E.; Chen, L.; Zhu, Q.; Chen, Z.; Wei, Y.; Zhang, W.; Dong, L.; Fang, W.; Chen, Z. Carbon Nitride with Single-Atom Nickel as Co-Catalyst for Visible-Light Promoted C—O Coupling. Chin. J. Chem. 2023, 41, 3281–3289.
- 44 Zhu, Q.; Zhao, E.; Shen, Y.; Chen, Z.; Fang, W. Photocatalytic C–N cross-coupling mediated by heterogeneous nickel-coordinated carbon nitride. Org. Biomol. Chem. 2023, 21, 4276–4281.
- 45 Yoshida, K.; Kon, K.; Shimizu, K.-i. Atomic-Resolution HAADF-STEM Study of Ag/Al2O3 Catalysts for Borrowing-Hydrogen and Acceptorless Dehydrogenative Coupling Reactions of Alcohols. Top. Catal. 2016, 59, 1740–1747.
- 46 Liu, P.; Qin, R.; Fu, G.; Zheng, N. Surface Coordination Chemistry of Metal Nanomaterials. J. Am. Chem. Soc. 2017, 139, 2122–2131.
- 47 Qin, R.; Liu, P.; Fu, G.; Zheng, N. Strategies for Stabilizing Atomically Dispersed Metal Catalysts. Small Methods 2018, 2, 1700286.
- 48 Yan, H.; Su, C.; He, J.; Chen, W. Single-atom catalysts and their applications in organic chemistry. J. Mater. Chem. A 2018, 6, 8793–8814.




