Atroposelective Synthesis of 2-Arylindoles via Chiral Phosphoric Acid-Catalyzed Direct Amination of Indoles†
Wen Bao
School of Pharmacy & State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, Gansu, 730000 China
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorYe-Hui Chen
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorYu-Wei Liu
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorShao-Hua Xiang
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Bin Tan
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorWen Bao
School of Pharmacy & State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, Gansu, 730000 China
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorYe-Hui Chen
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorYu-Wei Liu
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorShao-Hua Xiang
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Bin Tan
Shenzhen Grubbs Institute, Department of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Memory of Professor Xiyan Lu.
Comprehensive Summary
Indole-based atropisomers are a very important class of axially chiral compounds. However, the atroposelective synthesis of axially chiral 2-arylindole remains largely unexplored. In this study, we report the successful synthesis of atropisomeric 2-arylindoles using direct amination of indoles with p-quinonediimines in the presence of chiral phosphoric acid as a catalyst. Quinonediimine acts as an aminating reagent through formal polarity inversion of imine. The malonate group on the 2-aryl of 2-indoles was found to be essential for high enantioselectivity of the products. This could be due to the additional interaction between the ester group and the catalyst, as well as the intramolecular hydrogen bonding. Our findings provide a new strategy for the asymmetric construction of 2-arylindole atropisomers.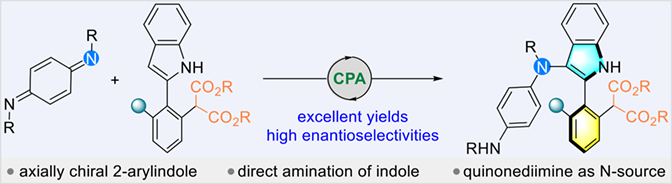
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300589-sup-0001-supinfo.pdfPDF document, 12.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Maehr, H.; Smallheer, J. Total syntheses of rivularins D1 and D3. J. Am. Chem. Soc. 1985, 107, 2943–2945.
- 2 Benincori, T.; Brenna, E.; Sannicoll, F.; Trimarco, L.; Antognazza, P.; Cesarotti, E.; Demartin, F.; Pilati, T.; Zotti, G. Chiral atropisomeric five-membered biheteroaromatic diphosphines: new ligands of the bibenzirnidazole and biindole series. J. Organomet. Chem. 1997, 529, 445–453.
- 3 Benincori, T.; Piccolo, O.; Rizzo, S.; Sannicoll, F. 3,3’-Bis(diphenylphosphino)-1,1’-disubstituted-2,2’-biindoles: easily accessible, electron-rich, chiral diphosphine ligands for homogeneous enantioselective hydrogenation of oxoesters. J. Org. Chem. 2000, 65, 8340–8347.
- 4 Jiang, F.; Luo, G.-Z.; Zhu, Z.-Q.; Wang, C.-S.; Mei, G.-J.; Shi, F. Application of naphthylindole-derived phosphines as organocatalysts in [4 + 1] cyclizations of o-quinone methides with Morita-Baylis-Hillman carbonates. J. Org. Chem. 2018, 83, 10060–10069.
- 5 Baumann, T.; Brgckner, R. Atropselective dibrominations of a 1,1’-disubstituted 2,2’-biindolyl with diverging point-to-axial asymmetric inductions. Deriving 2,2’-biindolyl-3,3’-diphosphane ligands for asymmetric catalysis. Angew. Chem. Int. Ed. 2019, 58, 4714–4719.
- 6 He, T.; Peng, L.; Li, S.; Hu, F.; Xie, C.; Huang, S.; Jia, S.; Qin, W.; Yan, H. Chiral naphthyl-C2-indole as scaffold for phosphine organocatalysis: application in asymmetric formal [4 + 2] cycloaddition reactions. Org. Lett. 2020, 22, 6966–6971.
- 7 Li, T.-Z.; Liu, S.-J.; Tan, W.; Shi, F. Catalytic asymmetric construction of axially chiral indole-based frameworks: an emerging area. Chem. Eur. J. 2020, 26, 15779–15792.
- 8 Cheng, J. K.; Xiang, S.-H.; Li, S.; Ye, L.; Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 2021, 121, 4805–4902.
- 9 Zhang, H.-H.; Shi, F. Organocatalytic atroposelective synthesis of indole derivatives bearing axial chirality: strategies and applications. Acc. Chem. Res. 2022, 55, 2562–2580.
- 10 He, X.-L.; Zhao, H.-R.; Song, X.; Jiang, B.; Du, W.; Chen, Y.-C. Asymmetric Barton-Zard reaction to access 3-pyrrole-containing axially chiral skeletons. ACS Catal. 2019, 9, 4374–4381.
- 11 Zhang, H.-H.; Wang, C.-S.; Li, C.; Mei, G.-J.; Li, Y.; Shi, F. Design and enantioselective construction of axially chiral naphthyl-indole skeletons. Angew. Chem. Int. Ed. 2017, 56, 116–121.
- 12 Jiang, F.; Chen, K.-W.; Wu, P.; Zhang, Y.-C.; Jiao, Y.; Shi, F. A strategy for synthesizing axially chiral naphthyl-indoles: Catalytic asymmetric addition reactions of racemic substrates. Angew. Chem. Int. Ed. 2019, 58, 15104–15110.
- 13 Qi, L.-W.; Mao, J.-H.; Zhang, J.; Tan, B. Organocatalytic asymmetric arylation of indoles enabled by azo groups. Nat. Chem. 2018, 10, 58–64.
- 14 Zhu, S.; Chen, Y.-H.; Wang, Y.-B.; Yu, P.; Li, S.; Xiang, S.-H.; Wang, J.-Q.; Xiao, J.; Tan, B. Organocatalytic atroposelective construction of axially chiral arylquinones. Nat. Commun. 2019, 10, 4268–4277.
- 15 Lu, D.-L.; Chen, Y.-H.; Xiang, S.-H.; Yu, P.; Tan, B.; Li, S. Atroposelective construction of arylindoles by chiral phosphoric acid-catalyzed cross-coupling of indoles and quinones. Org. Lett. 2019, 21, 6000–6004.
- 16 Ding, W.-Y.; Yu, P.; An, Q.-J.; Bay, K. L.; Xiang, S.-H.; Li, S.; Chen, Y.; Houk, K. N.; Tan, B. DFT-guided phosphoric-acid-catalyzed atroposelective arene functionalization of nitrosonaphthalene. Chem 2020, 6, 2046–2059.
- 17 Chen, Y.-H.; Li, H.-H.; Zhang, X.; Xiang, S.-H.; Li, S.; Tan, B. Organocatalytic enantioselective synthesis of atropisomeric aryl-p-quinones: Platform molecules for diversity-oriented synthesis of biaryldiols. Angew. Chem. Int. Ed. 2020, 59, 11374–11378.
- 18 Lu, S.; Ong, J.-Y.; Yang, H.; Poh, S. B.; Liew, X.; Seow, C. S. D.; Wong, M. W.; Zhao, Y. Diastereo- and atroposelective synthesis of bridged biaryls bearing an eight-membered lactone through an organocatalytic cascade. J. Am. Chem. Soc. 2019, 141, 17062–17067.
- 19 Yang, H.; Sun, H.-R.; He, R.-Q.; Yu, L.; Hu, W.; Chen, J.; Yang, S.; Zhang, G.-G.; Zhou, L. Organocatalytic cycloaddition of alkynylindoles with azonaphthalenes for atroposelective construction of indole-based biaryls. Nat. Commun. 2022, 13, 632–640.
- 20 Surgenor, R. R.; Liu, X.; Keenlyside, M. J. H.; Myers, W.; Smith, M. D. Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling. Nat. Chem. 2023, 15, 357–365.
- 21 Wang, J.-Y.; Sun, M.; Yu, X.-Y.; Zhang, Y.-C.; Tan, W.; Shi, F. Atroposelective construction of axially chiral alkene-indole scaffolds via catalytic enantioselective addition reaction of 3-alkynyl-2-indolylmethanols. Chin. J. Chem. 2021, 39, 2163–2171.
- 22 Sheng, F.-T.; Yang, S.; Wu, S.-F.; Zhang, Y.-C.; Shi, F. Catalytic asymmetric synthesis of axially chiral 3,3’-bisindoles by direct coupling of indole rings. Chin. J. Chem. 2022, 40, 2151–2160.
- 23 Wu, P.; Yu, L.; Gao, C.-H.; Cheng, Q.; Deng, S.; Jiao, Y.; Tan, W.; Shi, F. Design and synthesis of axially chiral aryl-pyrroloindoles via the strategy of organocatalytic asymmetric (2+3) cyclization. Fundam. Res. 2023, 3, 237–248.
- 24 Wang, H.-Q.; Wu, S.-F.; Yang, J.-R.; Zhang, Y.-C.; Shi, F. Design and organocatalytic asymmetric synthesis of indolyl-pyrroloindoles bearing both axial and central chirality. J. Org. Chem. 2023, 88, 7684–7702.
- 25 Da, B.-C.; Xiang, S.-H.; Li, S.; Tan, B. Chiral phosphoric acid catalyzed asymmetric synthesis of axially chiral compounds. Chin. J. Chem. 2021, 39, 1787–1796.
- 26 Cheng, J. K.; Xiang, S.-H.; Tan, B. Imidodiphosphorimidates (IDPis): catalyst motifs with unprecedented reactivity and selectivity. Chin. J. Chem. 2023, 41, 685–694.
- 27 Lin, X.; Wang, L.; Han, Z.; Chen, Z. Chiral spirocyclic phosphoric acids and their growing applications. Chin. J. Chem. 2021, 39, 802–824.
- 28 He, F.; Shen, G.; Yang, X. Asymmetric aminations and kinetic resolution of acyclic α-branched ynones. Chin. J. Chem. 2022, 40, 15–20.
- 29 Wang, G.-J.; Zhang, S.-Y.; Sun, Z.-L.; Li, P.; Ding, T.-M. Highly site- and enantioselective N-H functionalization of N-monosubstituted aniline derivatives affording pyrazolones bearing a quaternary stereocenter. Chin. J. Chem. 2022, 40, 1144–1148.
- 30 Xie, J.; Guo, Z.; Liu, W.; Zhang, D.; He, Y.-P.; Yang, X. Kinetic resolution of 1,2-diamines via organocatalyzed asymmetric electrophilic aminations of anilines. Chin. J. Chem. 2022, 40, 1674–1680.
- 31 Hu, Y.-L.; Wang, Z.; Yang, H.; Chen, J.; Wu, Z.-B.; Lei, Y.; Zhou, L. Conversion of two stereocenters to one or two chiral axes: atroposelective synthesis of 2, 3-diarylbenzoindoles. Chem. Sci. 2019, 10, 6777–6784.
- 32 Peng, L.; Li, K.; Xie, C.; Li, S.; Xu, D.; Qin, W.; Yan, H. Organocatalytic asymmetric annulation of ortho-alkynylanilines: synthesis of axially chiral naphthyl-C2-indoles. Angew. Chem. Int. Ed. 2019, 58, 17199–17204.
- 33 Jia, S.; Tian, Y.; Li, X.; Wang, P.; Lan, Y.; Yan, H. Atroposelective construction of nine-membered carbonate-bridged biaryls. Angew. Chem. Int. Ed. 2022, 61, e202206501.
- 34 Xu, D.; Huang, S.; Hu, F.; Peng, L.; Jia, S.; Mao, H.; Gong, X.; Li, F.; Qin, W.; Yan, H. Diversity-oriented enantioselective construction of atropisomeric heterobiaryls and N-aryl indoles via vinylidene ortho-quinone methides. CCS Chem. 2022, 4, 2686–2697.
- 35 Tian, M.; Bai, D.; Zheng, G.; Chang, J.; Li, X. Rh(III)-catalyzed asymmetric synthesis of axially chiral biindolyls by merging C-H activation and nucleophilic cyclization. J. Am. Chem. Soc. 2019, 141, 9527–9532.
- 36 He, Y.-P.; Wu, H.; Wang, Q.; Zhu, J. Palladium-catalyzed enantioselective Cacchi reaction: asymmetric synthesis of axially chiral 2,3-disubstituted indoles. Angew. Chem. Int. Ed. 2020, 59, 2105–2109.
- 37 Jacob, N.; Zaid, Y.; Oliveira, J. C. A.; Ackermann, L.; Wencel-Delord, J. Cobalt-catalyzed enantioselective C-H arylation of indoles. J. Am. Chem. Soc. 2022, 144, 798–806.
- 38 Shaaban, S.; Li, H.; Otte, F.; Strohmann, C.; Antonchick, A. P.; Waldmann, H. Enantioselective synthesis of five-membered-ring atropisomers with a chiral Rh(III) complex. Org. Lett. 2020, 22, 9199–9202.
- 39 Zou, Y.; Wang, P.; Kong, L.; Li, X. Rhodium-catalyzed atroposelective C-H arylation of (hetero)arenes using carbene precursors as arylating reagents. Org. Lett. 2022, 24, 3189–3193.
- 40 Yin, S.-Y.; Pan, C.; Zhang, W.-W. Scprh(III)-catalyzed enantioselective synthesis of atropisomers by C2-arylation of indoles with 1-diazonaphthoquinones. Org. Lett. 2022, 24, 3620–3625.
- 41 Liu, J.; Li, Q.; Shao, Y.; Sun, J. Atroposelective synthesis of axially chiral C2-arylindoles via rhodium-catalyzed asymmetric C-H bond insertion. Org. Lett. 2022, 24, 4670–4674.
- 42 Yu, L.; Liu, J.; Xiang, S.; Lu, T.; Ma, P.; Zhao, Q. Silver-catalyzed direct nucleophilic cyclization: enantioselective de novo synthesis of C−C axially chiral 2-arylindoles. Org. Lett. 2023, 25, 522–527.
- 43 Yao, C.-Z.; Xie, Z.-K.; Wang, J.-Y.; Zhang, J.-Y.; Zhao, Z.-Y.; Li, Q.; Yu, J. Stereogenic-at-cobalt(III) complex catalyzed halocyclization of alkynes: enantioselective access to axially chiral ortho-halo-C2-indoles. J. Org. Chem. 2023, 88, 6146–6158.
- 44 Qin, J.; Zhou, T.; Zhou, T.-P.; Tang, L.; Zuo, H.; Yu, H.; Wu, G.; Wu, Y.; Liao, R.-Z.; Zhong, F. Catalytic atroposelective electrophilic amination of indoles. Angew. Chem. Int. Ed. 2022, 61, e202205159.




