Synthesis of Planar-Chiral [2.2]Paracyclophane-Based Oxazole-Pyrimidine Ligands and Application in Nickel-Catalyzed 1,2-Reduction of α,β-Unsaturated Ketones
Juan Wang
School of Chemistry, Dalian University of Technology, 2 Linggong Road, Dalian, Liaoning, 116024 China
Search for more papers by this authorQing-Xian Xie
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Search for more papers by this authorXiang Li
School of Chemistry, Dalian University of Technology, 2 Linggong Road, Dalian, Liaoning, 116024 China
Search for more papers by this authorCorresponding Author
Chang-Bin Yu
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yong-Gui Zhou
School of Chemistry, Dalian University of Technology, 2 Linggong Road, Dalian, Liaoning, 116024 China
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorJuan Wang
School of Chemistry, Dalian University of Technology, 2 Linggong Road, Dalian, Liaoning, 116024 China
Search for more papers by this authorQing-Xian Xie
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
Search for more papers by this authorXiang Li
School of Chemistry, Dalian University of Technology, 2 Linggong Road, Dalian, Liaoning, 116024 China
Search for more papers by this authorCorresponding Author
Chang-Bin Yu
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yong-Gui Zhou
School of Chemistry, Dalian University of Technology, 2 Linggong Road, Dalian, Liaoning, 116024 China
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning, 116023 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
The planar-chiral ligands have been widely applied as a class of unique and significant ligands in asymmetric catalysis. Among them, chiral [2.2]paracycyclophane has emerged as a privileged type of planar-chiral framework and has been utilized as an important toolbox due to their structural stability. Herein, we design and synthesize [2.2]paracyclophane-derived oxazole-pyrimidine ligands (abberviated as PYMCOX). These N,N-ligands with stable properties, rigid structure and large steric hindrance performed successfully in nickel-catalyzed asymmetric 1,2-reduction of α,β-unsaturated ketones, affording the chiral allylic alcohols with up to 99% yield and 99% ee. Meanwhile, this reduction reaction could be conducted on gram-scale without loss of activity and enantioselectivity, and the chiral ligand could be conveniently recovered with high yield.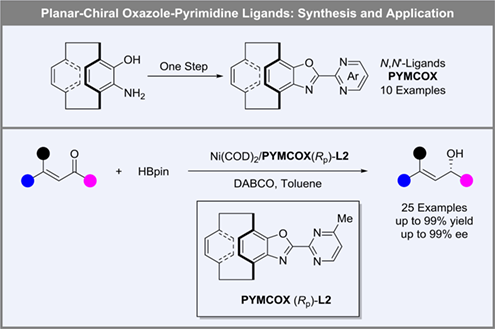
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300575-sup-0001-supinfo.pdfPDF document, 3.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Lumbroso, A.; Cooke, M. L.; Breit, B. Catalytic Asymmetric Synthesis of Allylic Alcohols and Derivatives and their Applications in Organic Synthesis. Angew. Chem. Int. Ed. 2013, 52, 1890–1932; (b) Trost, B. M.; Bartlett, M. J. Transition-Metal-Catalyzed Synthesis of Aspergillide B: An Alkyne Addition Strategy. Org. Lett. 2012, 14, 1322–1325.
- 2(a) Ohkuma, T.; Koizumi, M.; Doucet, H.; Pham, T.; Kozawa, M.; Murata, K.; Katayama, T.; Yokozawa, T.; Ikariya, T.; Noyori, R. Asymmetric Hydrogena-tion of Alkenyl, Cyclopropyl, and Aryl Ketones. RuCl2(xylbinap)(1,2-diamine) as a Precatalyst Exhibiting a Wide Scope. J. Am. Chem. Soc. 1998, 120, 13529–13530; (b) Arai, N.; Azuma, K.; Nii, N.; Ohkuma, T. Highly Enantioselective Hydrogenation of Aryl Vinyl Ketones to Allylic Alcohols Catalyzed by the Tol-Binap/Dmapen Ruthenium(II) Complex. Angew. Chem. Int. Ed. 2008, 47, 7457–7460; (c) Zhang, K.; Liu, Q.; He, R.; Chen, D.; Deng, Z.; Huang, N.; Zhou, H. Ruthenium-Catalysed Synthesis of Chiral Exocyclic Allylic Alcohols via Chemoselective Transfer Hydrogenation of 2-Arylidene Cycloalkanones. Green Chem. 2021, 23, 1628–1632.
- 3(a) Spogliarich, S.; Vidotto, S.; Farnetti, E.; Graziani, M. Highly Selective Catalytic Hydrogenation of Cyclic Enones. Tetrahedron: Asymmetry 1992, 3, 1001–1002; (b) Xie, J.-B.; Xie, J.-H.; Liu, X.-Y.; Kong, W.-L.; Li, S.; Zhou, Q.-L. Highly Enantioselective Hydrogenation of α-Arylmethylene Cyclo-alkanones Catalyzed by Iridium Complexes of Chiral Spiro Aminophosphine Ligands. J. Am. Chem. Soc. 2010, 132, 4538–4539; (c) Wang, Y.; Yang, G.; Xie, F.; Zhang, W. A Ferrocene- Based NH-Free Phosphine-Oxazoline Ligand for Iridium-Catalyzed Asymmetric Hydrogenation of Ketones. Org. Lett. 2018, 20, 6135–6139.
- 4(a) Pelšs, A.; Kumpulainen, E. T. T.; Koskinen, A. M. P. Highly Chemo-selective Copper-Catalyzed Conjugate Reduction of Stereo chemically Labile α,β-Unsaturated Amino Ketones. J. Org. Chem. 2009, 74, 7598–7601; (b) Moser, R.; Bošković, Ž. V.; Crowe, C. S.; Lipshutz, B. H. CuH-Catalyzed Enantioselective 1,2-Reductions of α,β-Unsaturated Ketones. J. Am. Chem. Soc. 2010, 132, 7852–7853; (c) Junge, K.; Wendt, B.; Addis, D.; Zhou, S.; Das, S.; Beller, M. Copper-Catalyzed Enantioselective Hydrosilylation of Ketones by Using Monodentate Binaphthophosphepine Ligands. Chem. Eur. J. 2010, 16, 68–73.
- 5(a) He, P.; Liu, X.; Zheng, H.; Li, W.; Lin, L.; Feng, X. Asymmetric 1,2-Reduction of Enones with Potassium Borohydride Catalyzed by Chiral N,N’-Dioxide-Scandium(III) Complexes. Org. Lett. 2012, 14, 5134–5137; (b) Szewczyk, M.; Stanek, F.; Bezłada, A.; Mlynarski, J. Zinc-Catalyzed Enantio-selective Hydrosilylation of Ketones and Imines under Solvent-Free Conditions. ChemCatChem 2016, 8, 3575–3579; (c) Vasilenko, V.; Blasius, C. K.; Wadepohl, H.; Gade, L. H. Mechanism-Based Enantiodivergence in Manganese Reduction Catalysis: A Chiral Pincer Complex for the Highly Enantioselective Hydroboration of Ketones. Angew. Chem. Int. Ed. 2017, 56, 8393–8397; (d) Sudo, A.; Yoshida, H.; Saigo, K. An Efficient Phosphorous-containing Oxazoline Ligand Derived from cis-2-Amino-3,3-dimethyl-indanol Application to Rhodium-Catalyzed Enantioselective Hydrosilylation of Ketones. Tetrahedron: Asymmetry 1997, 8, 3205–3208; (e) Wang, R.; Park, S. Recent Advances in Metal-Catalyzed Asymmetric Hydroboration of Ketones. ChemCatChem 2021, 13, 1898–1919.
- 6 Chen, F.; Zhang, Y.; Yu, L.; Zhu, S. Enantioselective NiH/Pmrox-Catalyzed 1,2-Reduction of α,β-Unsaturated Ketones. Angew. Chem. Int. Ed. 2017, 56, 2022–2025.
- 7For examples, see: (a) Cipiciani, A.; Fringuelli, F.; Piermatti, O.; Pizzo, F.; Ruzziconi, R. Asymmetric Diels-Alder, Michael, and Aldol Reactions Using a Planar Chiral 1,3-Oxazol-2(3H)-one Derived from (R)-(+)-4-Hydroxy[2.2]-paracyclophane. J. Org. Chem. 2002, 67, 2665–2670; (b) Friedmann, C. J.; Ay, S.; Bräse, S. Improved Synthesis of Enantiopure 4-Hydroxy[2.2]paracyclophane. J. Org. Chem. 2010, 75, 4612–4614.
- 8For examples, see: (a) Rozenberg, V.; Kharitonov, V.; Antonov, D.; Sergeeva, E.; Aleshkin, A.; Ikonnikov, N.; Orlova, S.; Belokon, Y. Scalemic 2-Formyl-3-hydroxy[2.2]paracyclophane: A New Auxiliary for Asymmetric Synthesis. Angew. Chem. Int. Ed. 1994, 33, 91–92; (b) Reich, H. J.; Cram, D. J. Macro Rings. XXXVI. Ring Expansion, Racemization, and Isomer Interconversions in the [2.2]Paracyclophane System through a Diradical Intermediate. J. Am. Chem. Soc. 1969, 91, 3517–3526.
- 9(a) Bai, Y.-Q.; Wang, X.-W.; Wu, B.; Wang, X.-Q.; Liao, R.-Z.; Li, M.; Zhou, Y.-G. Design and Synthesis of Planar-Chiral Oxazole-Pyridine N,N-Ligands: Application in Palladium-Catalyzed Asymmetric Acetoxylative Cyclization. ACS Catal. 2023, 13, 9829–9838; (b) Niu, T.; Liu, L.-X.; Wu, B.; Zhou, Y.-G. Synthesis of Tridentate PNO Ligands with Planar Chirality and Application in Iridium-Catalyzed Asymmetric Hydrogenation of Simple Ketones. J. Org. Chem. 2023, 88, 7863–7871.
- 10For examples, see: (a) Kopchuk, D. S.; Chepchugov, N. V.; Khasanov, A. F.; Kovalev, L. S.; Santra, S.; Nosova, E. V.; Zyryanov, G. V.; Majee, A.; Rusinov, V. L.; Chupakhin, O. N. A One-pot Approach to 10-(1H-1,2,3-Triazol-1-yl)pyrimido[1,2-α] Indoles via Aryne-mediated Transformations of 3-(Pyrimidin-2-yl)-1,2,4-triazines. Tetrahedron Lett. 2016, 57, 3862–3865; (b) Chen, X.; Cheng, A. C.; Connors, R. V.; Debenedetto, M. V.; Dransfield, P. J.; Fu, Z.; Harvey, J. S.; Heath, J. A.; Hedley, S. J.; Houze, J.; Judd, T. C.; Khakoo, A. Y.; Kopecky, D. J.; Lai, S.-J.; Ma, Z.; Nishimura, N.; Olson, S. H.; Pattaropong, V.; Swaminath, G.; Wang, X.; Yeh, W.-C. Heterocyclic Triazole Compounds Agonists of the Apj Receptor. US 2017/0320860, 2017.
- 11 Jimenez, N. H.; Li, G.; Doller, D.; Grenon, M.; White, A. D.; Ma, G.; Guo, M. Adamantyl Diamide Derivatives and Uses of Same. US 2010/0022546, 2010.
- 12 Takamasa, T.; Hajime, M.; Ayaka, H.; Shinya, N.; Yoshihiko, N. Preparation of Fused Heterocyclic Compound, Pest Control Agent Containing the Compound, and Pest Control Method. WO 2015/002211, 2015.
- 13(a) Bai, D.; Wu, F.; Chang, L.; Wang, M.; Wu, H.; Chang, J. Highly Regio- and Enantioselective Hydrosilylation of gem-Difluoroalkenes by Nickel Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202114918; (b) Sun, Y.; Guan, R.; Liu, Z.; Wang, Y. Recent Advances in Hydroboration of Alkenes Catalyzed by Fe, Co and Ni. Chin. J. Org. Chem. 2020, 40, 899–912.
- 14(a) Vasilenko, V.; Blasius, C. K.; Wadepohl, H.; Gade, L. H. Mechanism-Based Enantio-divergence in Manganese Reduction Catalysis: A Chiral Pincer Complex for the Highly Enantioselective Hydroboration of Ketones. Angew. Chem. Int. Ed. 2017, 56, 8393–8397; (b) Vasilenko, V.; Blasius, C. K.; Gade, L. H. One-Pot Sequential Kinetic Profiling of a Highly Reactive Manganese Catalyst for Ketone Hydroboration: Leveraging σ-Bond Metathesis via Alkoxide Exchange Steps. J. Am. Chem. Soc. 2018, 140, 9244–9254.




