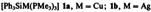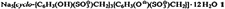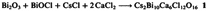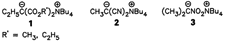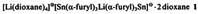Journal list menu
Export Citations
Download PDFs
Cover Picture (Angew. Chem. Int. Ed. Engl. 10/1988)
- First Published: October 1988

The title picture shows the luminescence of a Ni(CO)4 molecular beam after excitation with a xenon chloride UV laser. The pulsed molecular beam emerges from a nozzle (above in the picture, diameter ca. 50 μm) and expands conically. Interaction with the laser pulse leads to photolysis and formation of excited Ni(CO)3, which is responsible for the luminescence. In the center of the picture is the cone of a skimmer, the function of which is to admit only the core of the molecular beam through an opening (diameter < 1 mm) and thus collimate the beam. Such molecular beam experiments make it possible to investigate the physical-chemical basis of laser-activated gas-phase deposition of solids. Further, high-purity nickel films can be deposited on substrates in this way. More details on gas-phase deposition of thin metal and semiconductor films, as well as on high-quality powders for ceramics and on etching reactions for surface microstructuring, are reported by K. L. Kompa in a review article on p. 1314ff.
Graphical Abstract (Angew. Chem. Int. Ed. Engl. 10/1988)
- First Published: October 1988
Reviews
Electron Transfer and Charge Transfer: Twin Themes in Unifying the Mechanisms of Organic and Organometallic Reactions
- Pages: 1227-1266
- First Published: October 1988
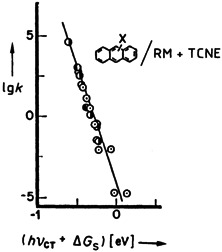
One mechanism is sufficient to describe numerous organic and organometallic reactions if the nucleophiles and electrophiles are also regarded as electron donors and electron acceptors, respectively. The resulting free energy relationship for electron transfer (FERET) makes it possible, for example, to describe such different reactions of TCNE as the cycloaddition to anthracene ( ) and the insertion into M-X bonds (⊙) by a common linear free energy relationship.
) and the insertion into M-X bonds (⊙) by a common linear free energy relationship.
Tumor Lectinology: At the Intersection of Carbohydrate Chemistry, Biochemistry, Cell Biology, and Oncology†
- Pages: 1267-1276
- First Published: October 1988
Lectins are sugar-binding proteins but neither sugar-specific enzymes nor antibodies. The natural targets of lectins are the sugar moieties of cellular glycoproteins, glycolipids, and proteoglycans; such structural elements might be involved in information transfer and storage. Tumors offer a model system with which to study lectins, especially in connection with neoglycoproteins. The latter are proteins that are coupled chemically with (in part quite complex) sugar derivatives. An additional advantage of the tumor model is its use to test diagnostic and therapeutic applications.
New Transition Metal Clusters with Ligands from Main Groups Five and Six†
- Pages: 1277-1296
- First Published: October 1988
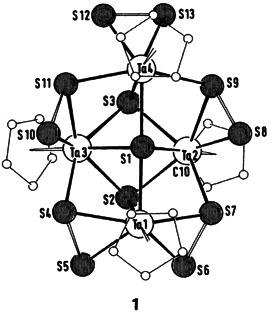
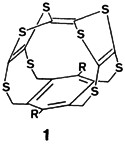
Low- and high-molecular-weight clusters having unusual structures and valence-electron concentrations are accessible by reactions between halide complexes and silylated chalcogen and pnictogen compounds. One example of the many clusters described is 1, in which sulfur displays three types of coordination. Such compounds provide information of importance to both solid-state and complex chemistry.
High Oxidation State Organometallic Chemistry, A Challenge—the Example of Rhenium†‡
- Pages: 1297-1313
- First Published: October 1988
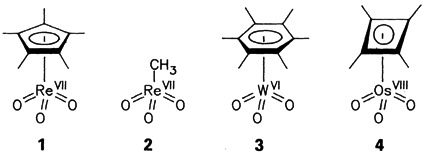
“By no means is it of merit to make an accidental discovery. Much more important is the recognition of possible consequences that might result from serendipity.” Trioxo(η5-pentamethylcyclopentadienyl)rhenium(VII) 1, prepared by chance several years ago, has proved to be a versatile compound, the methyl analogue of which, 2, has also borne fruit. These compounds have enhanced our understanding of olefin metathesis, the oxidative coupling of alkynes, and the oxidation of olefins. Perhaps it will soon be possible to synthesize the WO3 and OsO3 relatives, 3 and 4, respectively.
Laser Photochemistry at Surfaces—Laser-Induced Chemical Vapor Deposition and Related Phenomena†
- Pages: 1314-1325
- First Published: October 1988
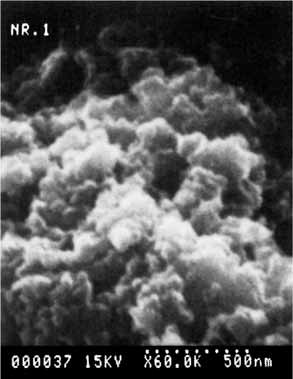
Laser chemistry can be employed in thin film deposition as well as in (structuring) ablation and etching. In general, reactions at surfaces can be initiated or assisted with lasers, as shown by the diverse examples in this article. One such possibility is the surface deposition of highly disperse powders, for example, silicon carbide, from the gas phase. Such powders are starting materials for high-performance ceramics.
Communications
[Au2Ag4L4]2⊖, [Au2Cu4L4]2⊖, [Au3Cu3L4]2⊖, [Cu4L3]2⊖, and [Ag9L6]3⊖ (L = ethane-1,2-dithiolate)—Novel Homoleptic Complexes of the Coinage Metals, including the First Heteronuclear Metal Thiolates†‡
- Pages: 1326-1329
- First Published: October 1988
![[Au2Ag4L4]2⊖, [Au2Cu4L4]2⊖, [Au3Cu3L4]2⊖, [Cu4L3]2⊖, and [Ag9L6]3⊖ (L = ethane-1,2-dithiolate)—Novel Homoleptic Complexes of the Coinage Metals, including the First Heteronuclear Metal Thiolates](/cms/asset/305f2a45-6f35-4948-ab5a-c926e00e08cc/must001.jpg)
The heteronuclear M6S8 cages A describe the structures of 1 and 2, which are formed from AgNO3 and CuCl2 or CuCl, respectively, ethanedithiolate (L), and [AuBr2]⊖. A homonuclear analogue of A is still unknown, since the combination of linear and trigonal-planar coordination is difficult to attain with a heavy element. The structure 3 can be regarded as a distorted sulfur icosahedron enclosing a likewise distorted silver cube with a further silver atom at the center.
Is the Molecule BOK Quasi-Linear?†‡
- Pages: 1329-1330
- First Published: October 1988
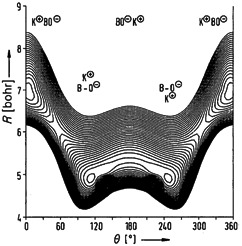
Most likely, yes—at least, that is the answer based on the results of SCF calculations. The interconversion of the two equivalent bent equilibrium structures via the linear structure BOK requires an activation energy of only 2 kJ mol−1, which is probably supplied by the zero-point vibrational energy.
Triazatrimetallabenzenes, a New Class of Inorganic Heterocycles; Synthesis and Structure of [Cp*TaN(Cl)]3†‡
- Pages: 1330-1331
- First Published: October 1988
π Complexes of an Amino-9-fluorenylideneborane†‡
- Pages: 1331-1337
- First Published: October 1988
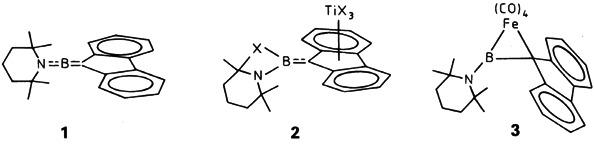
The amino(methylene)borane 1 can be complexed as an η2 and as an η5 ligand. Whereas TiCl4 coordinates in an η5 fashion to the five-membered ring (2) with transfer of a Cl atom to the B atom, reaction of 1 and Fe2(CO)9 gives compound 3 containing an FeBC ring; in 3, Fe is coordinated in a distorted trigonal-bipyramidal fashion.
Ethylenedithione (C2S2): Generation and Characterization by Neutralization-Reionization Mass Spectrometry†‡
- Pages: 1337-1339
- First Published: October 1988
The Structures of Perchloric Acid, HClO4, and Its Anhydride, Cl2O7†
- Pages: 1339-1341
- First Published: October 1988
Crystals of the title compounds contain practically isolated HClO4 molecules and Cl2O7 molecules best described as (ClO )2O2⊖. Solid perchloric acid can be described as rods of H-bridged molecules forming a closest rod packing (O-HċO: 82 and 203 pm, respectively), solid dichlorine heptoxide as cubic close-packed oxygen atoms, the neighboring tetrahedral gaps being occupied by chlorine atoms.
)2O2⊖. Solid perchloric acid can be described as rods of H-bridged molecules forming a closest rod packing (O-HċO: 82 and 203 pm, respectively), solid dichlorine heptoxide as cubic close-packed oxygen atoms, the neighboring tetrahedral gaps being occupied by chlorine atoms.
A New Approach to Ionic Ozonides†‡
- Pages: 1341-1342
- First Published: October 1988
Tetramethylammonium ozonide, N(CH3)4O3, is surprisingly stable! It forms red, transparent crystals, which only decompose above 75 °C. Till now, the thermally very labile alkali-metal ozonides were thought to be the most stable ionic ozonides. The possibility of developing a preparative chemistry involving ionic ozonides was therefore viewed with pessimism. N(CH3)4O3 was synthesized by double reaction of KO3 with N(CH3)4O2 in NH3. This route to ionic ozonides should prove to be general.
3- and 3,6-Silylated 2-Pyridinethiols: New Hindered Bidentate Ligands and Their Novel Silver and Copper Clusters†‡
- Pages: 1342-1344
- First Published: October 1988
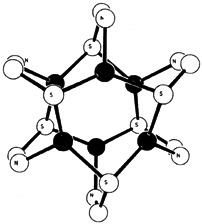
Analogous hexanuclear, paddlewheel complexes are formed by reaction of AgI and CuI precursors with the new ligand 3-tri-methylsilyl-2-pyridinethiol. MS2N coordination has thus been achieved for the first time (see right, • = metal). The large Me3Si group of the ligand prevents polymerization, which is observed for complexes of the parent compound. The ligand is accessible by silylation of 2-pyridinethiol.
Reactions of Pentacarbonyl(trichloromethyl isocyanide)chromium†‡
- Pages: 1344-1347
- First Published: October 1988
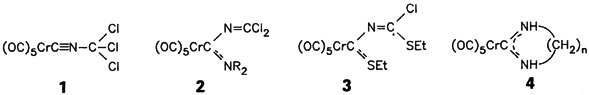
A broad palette of organometallic compounds can be synthesized from the readily accessible 1, which exhibits two mutually reinforcing electrophilic centers, the isocyano unit and the halogenated α-C atom. The high synthetic potential of 1, as well as its 2-azaalleniumylidene and dichlorocarbene nature, are exemplified by preparation of 2–4.
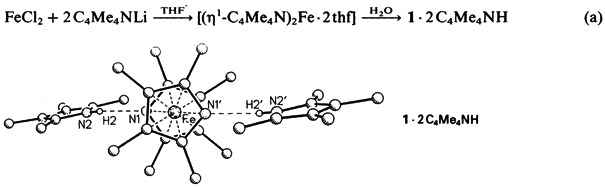
Tentacled Iron Sandwiches†‡
- Pages: 1347-1349
- First Published: October 1988
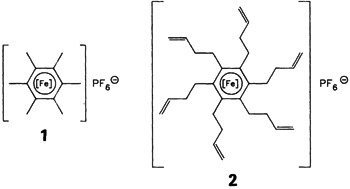
A sixfold alkyl chain extension, the reaction of 1 with tBuOK and H2CCHCH2Br in THF to give 2, was achieved in one step in quantitative yield. The FeII center of 2 can be completely and reversibly reduced but not oxidized (with Br2). Instead, bromine adds in a 1,3 fashion to all terminal CC bonds. The hexaalkylbenzene ligand can be released from 2 by irradiation. [Fe] = [(η5-C5H5)Fe]⊕.
Phosphane-Stabilized CuI and AgI Silanes†
- Pages: 1349-1350
- First Published: October 1988
First Direct Evidence for Nitrile Imine-Diazo Isomerization. Synthesis of Relatively Stable N-Silylated Nitrile Imines†‡
- Pages: 1350-1351
- First Published: October 1988
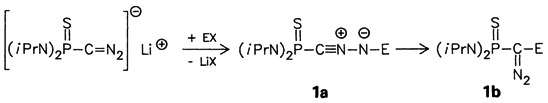
The title reaction 1a → 1b takes place between −78 and +55 °C, the rearrangement temperature depending on the substituents. The nitrile imines 1a can be characterized in solution by NMR and IR spectroscopy. Compounds 1a, E = SiMe3 and SiPh3, have also been trapped with methanol and with methyl acrylate (cycloaddition).
Reactivity of (R)-2-tert-Butyldihydrooxazole Derivatives Prepared from Serine and Threonine: Novel, Versatile Chiral Building Blocks†
- Pages: 1351-1353
- First Published: October 1988
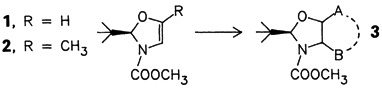
The unsaturated cyclic N,O-acetals 1 and 2 are promising starting materials for the synthesis of further valuable chiral intermediates. They were prepared from serine and threonine, respectively, by electrochemical decarboxylation. Acylation and addition reactions as well as catalytic hydrogenation were used to convert 1 and 2 into the mono-, bi-, and tricyclic compounds of type 3, in a regio- and stereoselective fashion.
A New Cubane Cluster with the [MoFe3S4]0 Core and Possible Relevance to the Fe3-Centers in Ferredoxins. The Synthesis, Structure and Properties of the [Fe3S4(SEt)3Mo(CO)3]3⊖ Anion†‡
- Pages: 1353-1355
- First Published: October 1988
![A New Cubane Cluster with the [MoFe3S4]0 Core and Possible Relevance to the Fe3-Centers in Ferredoxins. The Synthesis, Structure and Properties of the [Fe3S4(SEt)3Mo(CO)3]3⊖ Anion](/cms/asset/d6bd4382-5b09-433e-b094-b1b865b1bedd/must001.jpg)
The cluster anion 1 may be described as a weak complex of the fragment Mo(CO)3 and [Fe3S4(SEt)3]3⊖. An Fe oxidation state of + 2.67 was determined for 1 from the isomer shift in the Mössbauer spectrum. This finding indicates the presence of Mo0 in 1. In agreement with this conclusion are the positions of the CO stretching frequencies in the IR spectrum of 1.
Convenient Routes to Di-tert-butylsilanediyl: Chemical, Thermal and Photochemical Generation†‡
- Pages: 1355-1356
- First Published: October 1988
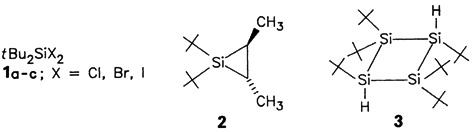
α-Elimination from 1 and thermal or photochemical cleavages of 2 produce tBu2Si:, the trapping or subsequent products of which can be isolated in good to very good yields. The silanediyl generated from 1a by α-elimination does not dimerize to tBu4Si2, but instead produces the four-membered ring compound 3 in 15% yield.
The Microwave Spectrum and Structure of the Ethylene–Sulfur Dioxide Complex†‡
- Pages: 1356-1358
- First Published: October 1988

The simplest π complex of SO2 with an organic molecule—C2H4 · SO2—was formed by ultrasonic expansion of a C2H4SO2Ne gas mixture. The structure derived from the microwave spectrum reveals that the SO2 and C2H4 planes are nearly parallel (Cs symmetry) and separated by 3.509 Å. The splitting pattern in the spectrum indicates the existence of a tunneling process between equivalent structures.
Solubilization of Elemental Sulfur in Water by Cationic and Anionic Surfactants†
- Pages: 1358-1359
- First Published: October 1988
The solubility of S8 in water is increased by a factor of 5000 when surfactants are added. This is surprising since elemental sulfur is especially hydrophobic and is neither wetted nor dissolved by water. The solubilization of S8 in aqueous surfactant solutions explains, for example, how sulfur bacteria are able to transport S8 into the cell, where it is oxidized to sulfate: the bacteria secrete surfactant molecules.
Novel Layer Structure of Sodium Calix[4]arenesulfonate Complexes—a Class of Organic Clay Mimics?†
- Pages: 1361-1362
- First Published: October 1988
![Novel Layer Structure of Sodium Calix[4]arenesulfonate Complexes—a Class of Organic Clay Mimics?](/cms/asset/a61ad5a9-336c-4f6d-81a6-ae72cd4acd8e/must001.jpg)
The crystalline sodium salt of calix[4]arene-sulfonate 1 consists of alternating organic and inorganic layers. Double layers of the basketlike pentaanions alternate with layers of sodium ions and water molecules; the similarity with the structures of clays is readily apparent. Acetone is bound in the cavity of 1, the O atom projecting into the hydrophilic layer (see right).
A Photolytic Entry into Oxepane Systems†
- Pages: 1362-1364
- First Published: October 1988
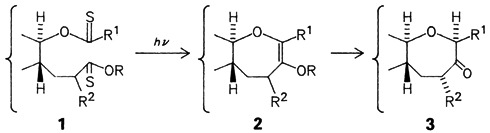
Saturated seven-membered rings containing an O atom—oxepanes— are found increasingly in nature. A new, highly promising strategy for the synthesis of oxepanes 3 starts from the dithionoesters 1, which, upon irradiation, cyclize with loss of S2 to give the olefins 2. Regioselective hydrolysis with tautomerization leads finally to the oxepanes 3.
Cs2Bi10Ca6Cl12O16: A New Type of Catalyst for Selective Oxidation Derived from Bismuth Oxychloride†
- Pages: 1364-1365
- First Published: October 1988
The title compound 1 is suitable for catalytic oxidative coupling of methane to give ethylene and ethane and for structural comparison with high-temperature superconductors. Compound 1 was obtained, as shown below, in an open crucible at 800°C. As catalyst, it leads to 40% ethylene and 12% ethane for 21% conversion. Compound 1 has an interesting layer structure. The Bi3⊕ and Ca2⊕ ions can occupy the same positions, but are not randomly distributed. Similar structures have been postulated for high-temperature super-conductors.
Solid-Phase Synthesis of O-Glycopeptide Sequences
- Pages: 1365-1367
- First Published: October 1988

4-Alkoxybenzyl alcohol resins are suitable supports for the solid-phase synthesis of O-glycopeptides. This was shown by the syntheses of the hexapeptides 1 and 2 in 55 and 48% yield, respectively, based on the first amino acid to be coupled. β-Elimination, racemization, or glycoside cleavage were not observed.
Tricyclo[3.1.0.02,6]hexanedione (the Valene of o-Benzoquinone), Bicyclo[2.1.1]hexane-2,3-dione, and Valenes of a Quinoxaline, of Phenazine, and of a Benzophenazine†
- Pages: 1369-1370
- First Published: October 1988
![Tricyclo[3.1.0.02,6]hexanedione (the Valene of o-Benzoquinone), Bicyclo[2.1.1]hexane-2,3-dione, and Valenes of a Quinoxaline, of Phenazine, and of a Benzophenazine](/cms/asset/e41fbb81-38ac-49af-b5ef-8da76caf9d07/must001.jpg)
The Swern oxidation is ideal for the transformation of strained cis-1,2-cyclo-pentanediols such as 1 to α-diketones such as 2. Compound 2, the valene of o-benzoquinone, is thus readily accessible. Its synthetic potential is demonstrated by the construction of other valenes, such as 3 (reaction of 2 with diaminomaleonitrile).
A Monomeric Diboryldilithiomethane with a 1,3-Diborataallene Structure†‡
- Pages: 1370-1372
- First Published: October 1988
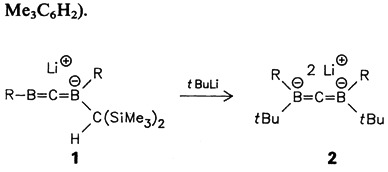
The title compound 2 is characterized by many interesting features: 2 is the first dilithiomethane stabilized by two boryl substituents; NMR and X-ray data establish its 1,3-diborataallene structure. In contrast to known dilithio-methane derivatives, which are aggregated, 2 is monomeric (R = 2,4,6-Me3C6H2).
Catalytic Syntheses of Functionalized Nitrogen Heterocycles from Cyanogen†‡
- Pages: 1372-1373
- First Published: October 1988
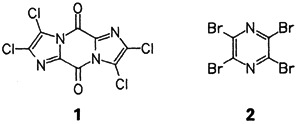
The synthesis of nitrogen heterocycles such as 1 and 2 from cyanogen and oxalyl halides is possible in one step and in yields of 60–70% with the catalyst system NR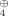 X⊖/HX, X = Cl, Br. Compound 1 can be easily cleaved to give functionalized imidazoles, and 2 is of interest owing to its bactericidal activity as well as its possible use as a synthetic building block.
X⊖/HX, X = Cl, Br. Compound 1 can be easily cleaved to give functionalized imidazoles, and 2 is of interest owing to its bactericidal activity as well as its possible use as a synthetic building block.
Metal-free Carbanion Salts as Initiators for the Anionic Polymerization of Acrylic and Methacrylic Acid Esters†
- Pages: 1373-1374
- First Published: October 1988
Molecular weights of up to 20 000 are attained in the polymerization of butyl acrylates with the malonates 1; the ratio of weight average molecular weight to number average molecular weight is ≤ 1.3. The likewise metal-free salts 2 and 3 also smoothly polymerize acrylic esters in THF or toluene at room temperature. The explanation for this behavior, which differs from that of the corresponding sodium or potassium salts, could be that metal ions are necessary as activators for the typical terminating reactions of anionic polymerization. Compounds 1–3 are readily accessible and may be stored for months at 0 °C.
5-Isopropyliden-1,2-oxathiole 2-Oxide by Photoisomerization of 2,2-Dimethyl-3(2H)-thiophenone 1-Oxide
- Pages: 1374-1375
- First Published: October 1988
The cyclic s-trans-1,3-dienes 3 are novel, highly promising synthetic intermediates. They may be obtained easily and selectively from the cyclic ketovinyl sulfoxides 2. This photoreaction is surprising, for the compounds 1 react completely differently upon irradiation. The photoisomerization of 2 presumably occurs via the diradical 4 as intermediate.
The p-Nitrocinnamyloxycarbonyl (Noc) Moiety—an Acid-stable Amino-protecting Group Removable under Neutral Conditions for Peptide and Glycopeptide Synthesis†
- Pages: 1375-1377
- First Published: October 1988
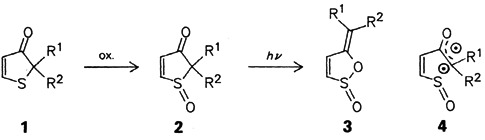
The bond between the new protecting group 1 and the amino groups of amino acids is very acid stable. However, it can be easily and quantitatively cleaved under almost neutral conditions by Pd0 catalysis. Further advantages of the protecting group 1 are its strong UV absorption and its stability in the RhI-catalyzed cleavage of “normal” allyl protecting groups; in addition, the amino acids containing these protecting groups are almost always crystalline.
A Donor Cage with Bent Tetrathiafulvalene Moiety†
- Pages: 1377-1379
- First Published: October 1988
Paraformaldehyde as Possible Chirality Amplifier†
- Pages: 1379-1381
- First Published: October 1988
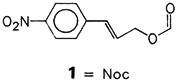
Can enantiomerically pure oligooxymethylene helices induce chirality by means of stereoelectronic effects? This question was investigated by use of the model compounds 1, n = 1–4. The asymmetric induction—determined by nucleophilic methylation—occurred in the same direction in all cases. This finding indicates that paraformaldehyde may have been involved in the amplification of chirality during the course of chemical evolution.
Solid State Structure of a Lithium Triorganostannate†
- Pages: 1381-1382
- First Published: October 1988
The first details of the coordination sphere of lithium triorganostannates have been obtained with lithium tris(α-furyl)stannate 1. The compound may be formulated as an ion pair. In the anion, the lithium ion exhibits an unusual, distorted octahedral oxygen coordination. The tin atoms are, as expected, coordinated in a pyramidal fashion.
A Novel Entry to the PC-Double Bond: the “Phospha-Wittig” Reaction
- Pages: 1382-1384
- First Published: October 1988
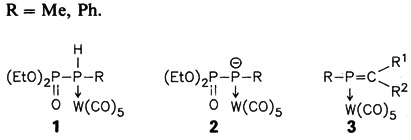
Phosphorylphosphane complexes 1 are the ideal starting compounds for the preparation of “phospha-Wittig” reagents 2, which react with aldehydes and ketones to give the corresponding phosphaalkene complexes 3. These can be isolated directly or after trapping with, for example, MeOH or cyclopentadiene. Liberation of the phosphaalkene from 3 should also be possible. R = Me, Ph.
Book Reviews
Book Review: An Introduction to Chemisorption and Catalysis by Metals. By R. P. H. Gasser
- Page: 1385
- First Published: October 1988
Book Review: Chemicals from Coal: New Processes (Series: Critical Reports on Applied Chemistry, Vol. 14). Edited by K. R. Payne
- Pages: 1385-1386
- First Published: October 1988
Book Review: Multinuclear NMR. Edited by J. Mason
- Pages: 1386-1387
- First Published: October 1988
Book Review: Inductively Coupled Plasmas in Analytical Atomic Spectrometry. Edited by A. Montaser and D. W. Golightly
- Pages: 1387-1388
- First Published: October 1988
Reviews
Intelligent Materials—A New Frontier
- Pages: 1389-1392
- First Published: October 1988
From Electronic/Ionic Conductors to Superconductors: Control of Materials Properties
- Pages: 1392-1400
- First Published: October 1988
Conference Report
Better High-Tech Ceramics by Improved Processing
- Pages: 1401-1403
- First Published: October 1988
Advanced Concepts for Ceramic Toughening
- Pages: 1403-1404
- First Published: October 1988
Research News
Book Reviews
Book Review: Corrosion of Materials: DECHEMA Corrosion Handbook: Corrosive Agents and their Interaction with Materials, Volume 1. Edited by D. Behrens
- Pages: 1407-1409
- First Published: October 1988
Book Review: Miscellanea: Photophysics of Polymers. Edited by Charles E. Hoyle and John M. Torkelson
- Page: 1409
- First Published: October 1988
Book Review: Physics at Surfaces. By A. Zangwill
- Pages: 1409-1410
- First Published: October 1988
Book Review: Metallic Superlattices—Artificially Structured Materials. Edited by T. Shinjo and T. Takada
- Page: 1410
- First Published: October 1988
Book Review: High-Resolution Soild-State NMR of Silicates and Zeolites. By G. Engelhardt and D. Michel
- Pages: 1410-1411
- First Published: October 1988
Book Review: Inorganic Thermochromism. By K. Sone and Y. Fukuda
- Page: 1411
- First Published: October 1988






![Triazatrimetallabenzenes, a New Class of Inorganic Heterocycles; Synthesis and Structure of [Cp*TaN(Cl)]3](/cms/asset/9c72e946-2f2d-42ab-a6c0-73ddbe4f1a2f/must001.jpg)
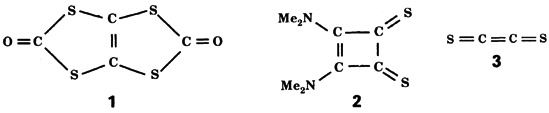
 can be neutralized in a collision experiment to give 3, whose existence has been predicted theoretically.
can be neutralized in a collision experiment to give 3, whose existence has been predicted theoretically.