3- and 3,6-Silylated 2-Pyridinethiols: New Hindered Bidentate Ligands and Their Novel Silver and Copper Clusters†‡
Corresponding Author
Prof. Eric Block
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)Search for more papers by this authorMichael Gernon
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)
Search for more papers by this authorHyunkyu Kang
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)
Search for more papers by this authorCorresponding Author
Prof. Jon Zubieta
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)Search for more papers by this authorCorresponding Author
Prof. Eric Block
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)Search for more papers by this authorMichael Gernon
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)
Search for more papers by this authorHyunkyu Kang
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)
Search for more papers by this authorCorresponding Author
Prof. Jon Zubieta
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)
Department of Chemistry, State University of New York at Albany, Albany, New York 12222 (USA)Search for more papers by this authorThis work was supported by the National Institutes of Health, the National Science Foundation, the Petroleum Research Fund, administered by the American Chemical Society, the Herman Frasch Foundation, and the Société Nationale Elf Aquitaine.
Dedicated to Professor Hans Bock on the occasion of his 60th birthday
Graphical Abstract
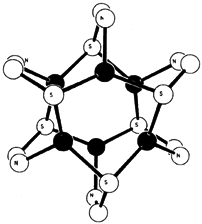
Analogous hexanuclear, paddlewheel complexes are formed by reaction of AgI and CuI precursors with the new ligand 3-tri-methylsilyl-2-pyridinethiol. MS2N coordination has thus been achieved for the first time (see right, • = metal). The large Me3Si group of the ligand prevents polymerization, which is observed for complexes of the parent compound. The ligand is accessible by silylation of 2-pyridinethiol.
References
- 1(a) I. G. Dance, Polyhedron 5 (1986) 1037; (b) P. G. Blower, J. R. Dilworth, Coord. Chem. Rev. 76 (1987) 121.
- 2 E. Block, M. Aslam, Tetrahedron 44 (1988) 281, and references cited therein.
- 3 S. A. Koch, R. Fitkar, M. Miller, T. O'Sullivan, Inorg. Chem. 24 (1984) 122.
- 4 B. K. Koo, E. Block, S. Liu, J. Zubieta, Polyhedron 7 (1988) 1397; E. Block, V. Eswarakrishnan, M. Gernon, G. Ofori-Okai, C. Saha, K. Tang, J. Zubieta, J. Am. Chem. Soc., in press; E. Block, M. Gernon, H. Kang, S. Liu, J. Zubieta, J. Chem. Soc. Chem. Commun. 1988, 1031.
- 5 K. Tang, M. Aslam, E. Block, T. Nicholson, J. Zubieta, Inorg. Chem. 27 (1987) 1488.
- 6 6a: 55%; GC-MS: m/z 183 (M⊕, 14%), 168 (M⊕ −15, 100%); 1H NMR (CDCl3): δ = 17.59 (d, 1H, J = 7.2 Hz), 7.56 (d, 1H, J = 6.4 Hz), 6.76 (t, 1H, J = 6.6 Hz), 0.42 (s, 9H); 13C NMR (CDCl3): δ = 181.00 (C), 144.55 (CH), 144.10 (C), 137.13 (CH), 113.57 (CH), −1.31 (CH3); IR (KBr): ṽ = 2850 (br), 1600, 1570, 1320, 1240, 1140, 1040, 850, 750 cm−1. 6b: 45%; m.p. 135–136°C (sublimed 130°C/0.1 torr); GC-MS: m/z 225 (M⊕, 3%), 196 (10%), 168 (33%), 140 (37%); 1H NMR (CDCl3): δ = 7.56–7.52 (m, 2H), 6.74 (t, 1H, J ≅ 6.6 Hz), 1.07–0.92 (m, 15H); 13C NMR (CDCl3): δ = 81.35 (C), 145.80 (CH), 141.71 (C), 137.02 (CH), 113.31 (CH), 7.54, 2.67; IR (KBr): ṽ = 2900 (br), 1570, 1300, 1150, 1010, 730 cm−1. 6c: 36%; m.p. 161–162°C (sublimed at 150°C/0.1 torr); GC-MS: m/z 245 (M⊕, 21%), 230 (70%), 196 (39%), 168 (M⊕ −77, 100%), 167 (M⊕ −78, 96%), 152 (39%); 1H NMR (CDCl3): δ = 7.61–7.58 (m, 2H), 7.46 (dd, 1H, J≅6.5, 2.1 Hz), 7.38–7.35 (m, 3H), 7.32 (dd, 1H, J = 7.6, 2.1 Hz), 6.61 (t, 1H, J=6.6 Hz), 0.74 (s, 6H); 13C NMR (CDCl3): δ = 181.09 (C), 146.00 (CH), 142.54 (C), 137.57 (C), 137.46 (CH), 134.42 (CH), 129.11 (CH), 127.84 (CH), 113.40 (CH), −2.79 (CH3); IR (KBr): ṽ = 2850, 1605, 1580, 1430, 1305, 1150 cm−1. 7a: 52%; m.p. 174–175°C (sublimed at 165°C/0.1 mm); GC-MS: m/z 339 (M⊕, 4%), 282 (100%), 224 (57%), 210 (21%), 73 (38%), 57 (42%); 1H NMR (CDCl3): δ = 10.4 (br s, 1H), 7.46 (d, 1H, J = 6.8 Hz), 6.72 (d, 1H, J = 6.8 Hz), 1.00 (s, 9H), 0.95 (s, 9H), 0.41 (s, 6H), 0.33 (s, 6H); 13C NMR (CDCl3): δ = 184.58 (C), 152.52 (C), 143.94 (CH), 142.69 (C), 119.84 (CH), 27.90 (CH3), 26.24 (CH3), 18.32 (C), 16.87 (C), −3.80 (CH3), −7.16 (CH3); IR (KBr): ṽ = 2950, 1570, 1550, 1470, 1290 cm−1. 7b: 37%; m.p. 193–194°C; CI-MS: m/z 587 (M⊕); 1H NMR (CDCl3): δ = 10.4 (br s, 1H), 7.60–7.30 (m, 21H), 6.85 (d, 1H, J = 6.7 Hz), 1.33 (s, 9H), 1.22 (s, 9H); 13 C NMR (CDCl3): δ = 185.34 (C), 150.45 (C), 146.83 (CH), 141.33 (C), 137.17 (CH), 136.11 (CH), 134.96 (C), 130.81 (CH), 129.71 (C), 128.94 (CH), 128.70 (CH), 127.56 (CH), 121.25 (CH), 30.65 (CH3), 28.34 (CH3), 19.08 (C), 18.84 (C); IR (KBr): ṽ = 3325, 2950, 1540, 1430, 1295, 1150, 1140, 1110, 740, 700 cm−1. 7c: 28%; m.p. 126–127°C (sublimed at 120°C/0.1 mm); GC-MS: m/z 297 (M⊕, 34%), 282 (M⊕ −15, 100%), 252 (42%), 224 (27%), 196 (38%), 73 (26%), 59 (33%); 1H NMR (CDCl3): δ = 10.7 (br. s, 1H), 7.44 (d, 1H, J = 6.5 Hz), 6.75 (dd, 1H, J = 6.5, 2.5 Hz), 1.00 (t, 9H, J = 7.4 Hz), 0.86 (q, 6H, J = 7.4 Hz), 0.40 (s, 9H); 13C NMR (CDCl3): δ = 183.98 (C), 152.40 (C), 144.10 (C), 142.35 (CH), 120.32 (CH), 7.04 (CH3), 2.32 (CH2), −1.39 (CH3); IR (KBr): ṽ = 3120, 3000, 2850, 2800, 1580, 1560, 1300, 1250, 1160, 875, 860, 740 cm−1. 8: 1H NMR (CD2Cl2): δ = 7.63 (d, 1H), 7.25 (d, 1H), 6.47 (t, 1H), 0.43 (s, 9H). 9: 1H NMR (CD2Cl2): δ = 7.55 (d, 1H), 7.20 (d, 1H), 6.45 (t, 1H), 0.46 (s, 9H), Satisfactory elemental analyses were obtained for all new compounds.
- 7(a) D. L. Comins, D. H. LaMunyon, Tetrahedron Lett. 29 (1988) 773; (b) A. Wright, R. West, J. Am. Chem. Soc. 96 (1974) 3222.
- 8 8 · CH2Cl2: monoclinic, space group A2/m, a = 10.968(2), b = 20.123(4), c = 16.779(3) Å, β = 96.45(1)°, V = 3679.9(12) Å3, Z = 2, ρcalcd = 1.33 g cm−3; structure solution and refinement based upon 1787 reflections with F0≥6σ(F0)(MoKα, λ = 0.71073 Å), R = 0.0530, Rw = 0.0583 (192 variables). Cu2 and Cu2a and the associated ligand groups occupy the crystallographic mirror plane; the twofold axis normal to this plane and passing between the Cu3S3 rings generates other symmetry related positions 9 · CH2Cl2: isomorphous with 8, space group C2/m, a = 17.201(3), b = 20.61(3), c = 10.977(2) Å, β = 95.46(1)°, V = 3873.8(13)Å3, Z = 2, ρcalcd = 1.49 g cm−3; structure solution and refinement based upon 2260 reflections with F0≥6σ(F0) (MoKα, λ = 0.71073 Å), R = 0.0561, Rw = 0.0654 (189 variables). Both structures present a CH2Cl2 molecule of crystallization disordered about a mirror plane. Structural data were collected on a Nicolet R3m/V diffractometer at 23°C, using a graphite monochromator. All non-hydrogen atoms were refined anisotropically for both 8 and 9.
- 9
P. M. Keehn in
P. M. Keehn,
S. M. Rosenfeld (Eds):
Cyclophanes, Vol. I,
Academic Press, New York
1983, pp. 69–239.
10.1016/B978-0-12-403001-5.50009-0 Google Scholar
- 10 G. A. Bowmaker, L.-C. Tan, Aust. J. Chem. 32 (1979) 1443.
- 11 I. G. Dance, J. Chem. Soc. Chem. Commun. 1976, 68.
- 12 G. A. Bowmaker, G. R. Clark, J. K. Seadon, I. G. Dance, Polyhedron 3 (1984) 535.
- 13 I. G. Dance, Aust. J. Chem. 31 (1978) 2195.
- 14 S. G. Rosenfield, H. P. Buends, L. Gilmini, D. W. Stephen, P. K. Mascharak, Inorg. Chem. 26 (1987) 2792.
- 15 A. J. Deeming, K. I. Hardcastle, M. N. Meah, P. A. Bates, H. M. Dawes, M. B. Hursthouse, J. Chem. Soc. Dalton Trans. 1988, 227; A. J. Deeming, M. N. Meah, P. A. Bates, M. B. Hursthouse, J. Chem. Soc. Dalton Trans. 1988, 235, and references cited therein.