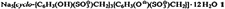Novel Layer Structure of Sodium Calix[4]arenesulfonate Complexes—a Class of Organic Clay Mimics?†
This work was supported by the U. S. National Science Foundation.
Graphical Abstract
The crystalline sodium salt of calix[4]arene-sulfonate 1 consists of alternating organic and inorganic layers. Double layers of the basketlike pentaanions alternate with layers of sodium ions and water molecules; the similarity with the structures of clays is readily apparent. Acetone is bound in the cavity of 1, the O atom projecting into the hydrophilic layer (see right).