Journal list menu
Export Citations
Download PDFs
Cover Picture: Molecular Mousetraps: Gas-Phase Studies of the Covalent Coupling of Noncovalent Complexes Initiated by Reactive Carbenes Formed by Controlled Activation of Diazo Precursors (Angew. Chem. Int. Ed. 9/2003)
- Page: 957
- First Published: 26 February 2003
Molecular mousetraps are an unprecedented class of host–guest reagents. In the example shown in the cover picture, 18-crown-6 ether is utilized as the “bait” to attract target molecules with a protonated primary amine functional group. Appropriate activation of the noncovalent complex springs the diazo “trap”, which leads to covalent attachment of the two molecules depicted in the reaction scheme. The research is detailed in the Communication by J. L. Beauchamp, B. M. Stoltz et al. on page 1012 ff.
Graphical Abstract: Angew. Chem. Int. Ed. 9/2003
- Pages: 960-967
- First Published: 26 February 2003
Book Review: Envisioning Science The Design and Craft of the Science Image. By Felice Frankel
- Pages: 969-979
- First Published: 26 February 2003
Book Review: Phosphorus–Carbon Heterocyclic Chemistry: The Rise of a New Domain Edited by François Mathey
- Page: 970
- First Published: 26 February 2003
Book Review: Nitrogen, Oxygen and Sulfur Ylide Chemistry Edited by J. Stephen Clark
- Page: 971
- First Published: 26 February 2003
Möbius Strips of NbSe3: Morphology Design and Solid-State Chemistry
- Pages: 972-974
- First Published: 26 February 2003
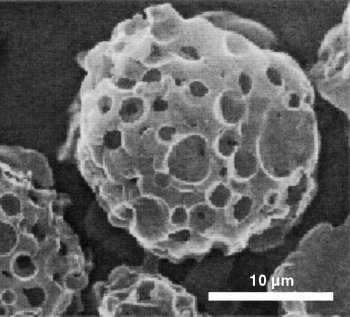
A preparative challenge in inorganic solid-state synthesis is to control the morphology of the arising products, for example, of vaterite microsponges (see picture). An important breakthrough in this area has now been achieved with the preparation of microcrystalline Möbius strips and rings from niobium selenide. The synthesis was achieved by a versatile combination of chemical transport reactions and templating techniques.
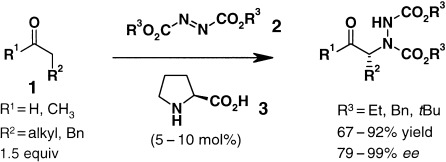
Proline-Catalyzed Asymmetric α-Amination of Aldehydes and Ketones—An Astonishingly Simple Access to Optically Active α-Hydrazino Carbonyl Compounds
- Pages: 975-978
- First Published: 26 February 2003
Organic Templates for the Generation of Inorganic Materials†
- Pages: 980-999
- First Published: 26 February 2003
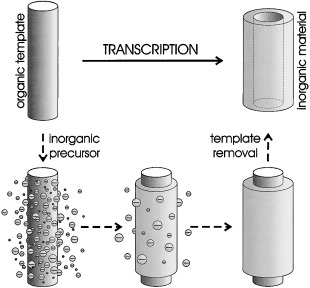
Shapely structured inorganic materials of diverse morphologies and compositions are nowadays obtainable through the transcription of organic templates (see picture). This review gives an overview of the various kinds of templates, precursors, and methods that have been employed for this purpose, as well as the diversity of inorganic materials that have been obtained.
Metallacarborane-Based Nanostructures: A Carbon-Wired Planar Octagon†
- Pages: 1002-1005
- First Published: 26 February 2003
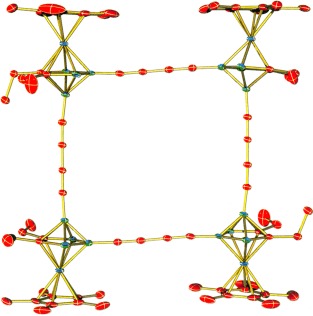
A planar C16B8 octagonal macrocycle that incorporates four identical metallacarborane units, linked by four diethynyl chains, has been prepared from a monomeric cobaltacarborane through a designed multistep synthesis (see picture). The air-stable molecule undergoes stepwise electrochemical reduction in THF solution to generate mono-, di-, and tetraanions, with the cyclic and square-wave voltammetric data indicating significant electron delocalization in the ring system.
Preparation of Artificial Metalloenzymes by Insertion of Chromium(III) Schiff Base Complexes into Apomyoglobin Mutants†
- Pages: 1005-1008
- First Published: 26 February 2003
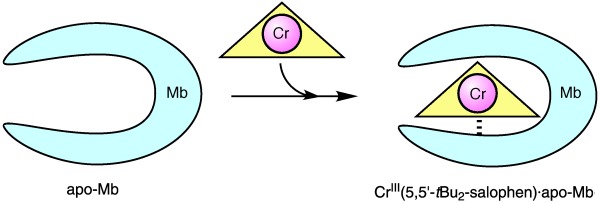
Insertion of a symmetric metal complex, [CrIII(5,5′-tBu-salophen)]+ (H2salophen=N,N′-bis(salicylidene)-1,2-phenylenediamine), into the active site of apomyoglobin is demonstrated (see picture). The metal ion and the ligand structure are very important factors that influence the binding affinity of the metal complex with the myoglobin (Mb) cavity. Semisynthetic metalloenzymes can catalyze enantioselective sulfoxidation by using the chiral protein cavity.
Oligonucleotide-Templated Self-Assembly of Nucleotide Bolaamphiphiles: DNA-Like Nanofibers Edged by a Double-Helical Arrangement of A–T Base Pairs
- Pages: 1009-1012
- First Published: 26 February 2003
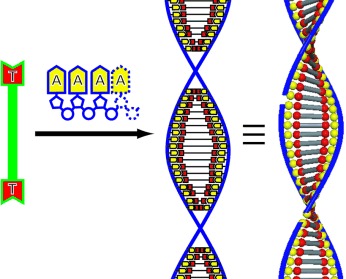
A stack with a twist is formed when a thymidine bolaamphiphile (green rod with red ends in the scheme) is allowed to interact with strands of oligoadenylic acid (purple chain with yellow pentagons) in aqueous solution. Complementary A–T hydrogen bonding leads to nanofibers having a DNA-like double helix.
Molecular Mousetraps: Gas-Phase Studies of the Covalent Coupling of Noncovalent Complexes Initiated by Reactive Carbenes Formed by Controlled Activation of Diazo Precursors†
- Pages: 1012-1015
- First Published: 26 February 2003
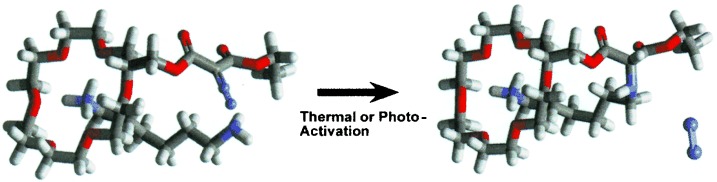
The covalent linking of noncovalently bound host–guest complexes in the gas phase is achieved by the formation of a highly reactive carbene from the host molecule, which is generated by collision-activated dissociation. Possible mechanisms for the resulting intermolecular reactions are explored by theory and experiment.
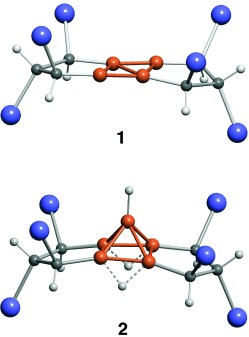
A Rare-Earth Metal TCNQ Magnet: Synthesis, Structure, and Magnetic Properties of {[Gd2(TCNQ)5(H2O)9][Gd(TCNQ)4(H2O)3]}⋅4 H2O†
- Pages: 1015-1018
- First Published: 26 February 2003
![A Rare-Earth Metal TCNQ Magnet: Synthesis, Structure, and Magnetic Properties of {[Gd2(TCNQ)5(H2O)9][Gd(TCNQ)4(H2O)3]}⋅4 H2O](/cms/asset/ab7e4df1-e401-49dd-93fb-553123fec59a/mcontent.jpg)
A lanthanide/organic radical based molecular magnet (Tc=3.5 K) has been prepared from a reaction of GdCl3⋅6 H2O with Li[TCNQ] (TCNQ=7,7,8,8-tetracyanoquinodimethane). The title compound was structurally characterized by single-crystal X-ray diffraction and contains distinct anionic and cationic (see picture) networks containing Gd, TCNQ, and H2O in different ratios.
Direct Liquid-Phase Sulfonation of Methane to Methanesulfonic Acid by SO3 in the Presence of a Metal Peroxide†
- Pages: 1019-1021
- First Published: 26 February 2003
Control of α/β Stereoselectivity in Lewis Acid Promoted C-Glycosidations Using a Controlling Anomeric Effect Based on the Conformational Restriction Strategy†
- Pages: 1021-1023
- First Published: 26 February 2003
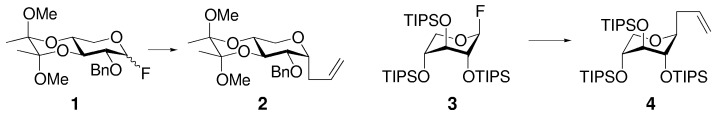
The kinetic anomeric effect has been used to control both α and β stereoselectivity in glycosidation reactions. Depending on the restricted conformation 4C1 or 1C4 of the substrate, the anomeric α (1→2) or β product (3→4) was obtained highly stereoselectively from the Lewis acid promoted anomeric allylation with allyltrimethylsilane (TIPS=triisopropylsilyl).
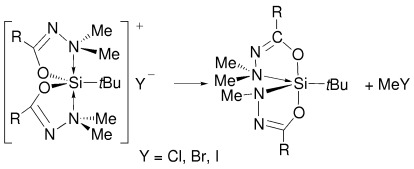
An Unexpected, Sterically Driven, Methyl Halide Elimination in Pentacoordinate Siliconium Halide Salts: Silicon Complexes with Equatorial Nitrogen Coordination†
- Pages: 1023-1026
- First Published: 26 February 2003
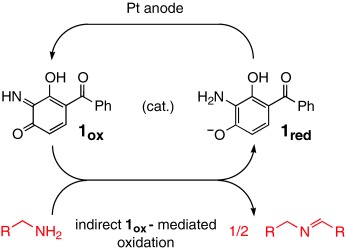
Oxidation of Unactivated Primary Aliphatic Amines Catalyzed by an Electrogenerated 3,4-Azaquinone Species: A Small-Molecule Mimic of Amine Oxidases
- Pages: 1026-1029
- First Published: 26 February 2003
Efficient Degradation of Organic Pollutants by Using Dioxygen Activated by Resin-Exchanged Iron(II) Bipyridine under Visible Irradiation†
- Pages: 1029-1032
- First Published: 26 February 2003
A Highly Enantioselective Catalyst for the Asymmetric Nozaki–Hiyama–Kishi Reaction of Allylic and Vinylic Halides†
- Pages: 1032-1035
- First Published: 26 February 2003
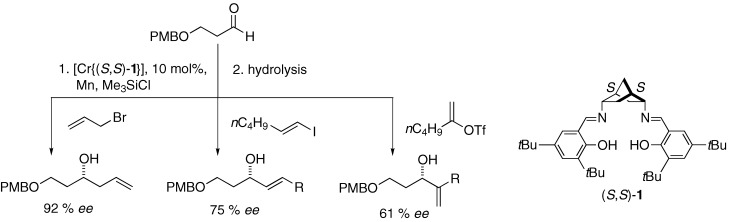
Catalytic asymmetric addition of vinylic halides and triflates to aldehydes, with useful levels of stereoinduction, has been achieved for the first time using the salen-type ligand (S,S)-5 (see scheme; PMB=para-methoxybenzyl), which contains the novel endo,endo-2,5-diamino norbornane building block. This asymmetric Nozaki–Hiyama–Kishi reaction leads to the coupling of various halides and aldehydes with high yields and enantioselectivities (up to 92 % ee).
A Catalytic Carbon–Phosphorus Ylide Reaction: Phosphane-Catalyzed Annulation of Allylic Compounds with Electron-Deficient Alkenes†
- Pages: 1035-1037
- First Published: 26 February 2003
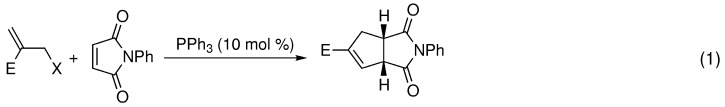
Readily available starting materials and high selectivity: These are two appealing features of the phosphane-catalyzed carbon–phosphorus ylide reaction described. Bromide, acetate, or tert-butyl carbonate derivatives of adducts formed by the Morita–Baylis–Hillman reaction react with PPh3 to form an allylic ylide. Phosphane-catalyzed annulation of the ylides with electron-deficient alkenes results in the facile construction of cyclopentenes [Eq. (1)]. X=Br, OAc, OBoc; E=CO2Et.
Stabilization of Low-Oxidation-State Early Transition-Metal Complexes Bearing 1,2,4-Triphosphacyclopentadienyl Ligands: Structure of [{Sc(P3C2tBu2)2}2]; ScII or Mixed Oxidation State?
- Pages: 1038-1041
- First Published: 26 February 2003
![Stabilization of Low-Oxidation-State Early Transition-Metal Complexes Bearing 1,2,4-Triphosphacyclopentadienyl Ligands: Structure of [{Sc(P3C2tBu2)2}2]; ScII or Mixed Oxidation State?](/cms/asset/e9de3786-f2a2-4fad-abfd-e2340f895146/mcontent.jpg)
ScII or mixed oxidation state ScIII/ScI? Reduction of the homoleptic ScIII complex [Sc(P3C2tBu2)3] allows access to the subvalent [{Sc(P3C2tBu2)2}2] complex (see picture), in which the metal centers are formally bivalent. DFT calculations support fomulation of the reduced species as a mixed oxidation state ScIII/ScI complex.
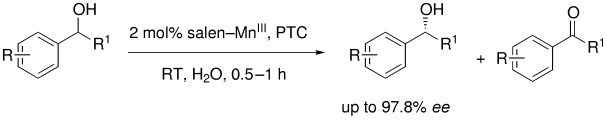
Chiral-Mn(Salen)-Complex-Catalyzed Kinetic Resolution of Secondary Alcohols in Water†
- Pages: 1042-1044
- First Published: 26 February 2003
Protonation of Cubane in the Gas Phase: A High-Level Ab Initio and DFT Study†
- Pages: 1044-1046
- First Published: 26 February 2003
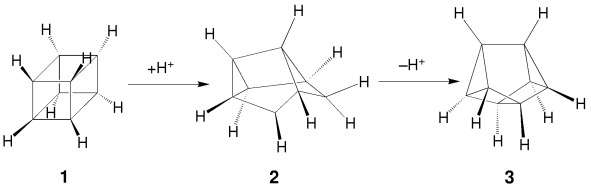
The tetracyclo[4.2.0.02,4.03,8] oct-7-ylium cation is the protonation product of cubane (1) in the gas phase according to ab initio and DFT calculations. At the G2(MP2) level, the calculated gas-phase basicity of 1 is about 60 kcal mol−1 larger than that determined experimentally. The experimental thermodynamics are consistent with fast deprotonation of 2 to give cuneane (3).
Synthesis and Structure of Cyclic Gold(I) Phosphanyl Complexes [{Au(PR2)}n]†
- Pages: 1046-1048
- First Published: 26 February 2003
![Synthesis and Structure of Cyclic Gold(I) Phosphanyl Complexes [{Au(PR2)}n]](/cms/asset/d66bdbc1-e620-4bd9-a05b-fac52b69be49/mcontent.jpg)
Gold(I) Phosphanyl Complexes, such as [Au(PR2)]n, have been known for more than 25 years. However, the solid-state structure of such complexes, as well as their nature in solution, has remained somewhat of a mystery. The figure displays the cyclic and polymeric forms that are possible (L=endgroup). The structures of the complexes, and the occurrence of Au⋅⋅⋅Au interactions are discussed.
Topomerization of a Distorted Diamond-Shaped Tetraborane(4) and Its Hydroboration to a closo-Pentaborane(7) with a nido Structure†
- Pages: 1049-1052
- First Published: 26 February 2003
The exchange of the short and long diagonal of the B4 diamond of 1 is the simplest variant to date of the diamond–square–diamond rearrangement, which plays a central role in the isomerization of polyhedral boranes, carboranes, and metallaboranes. Compound 2, which is readily accessible from 1, is the first derivative of pentaborane(7).
The Fluoroacyltris(trifluoromethyl)borate Ion, [(CF3)3BC(O)F]−, a Fluoroacylboron Complex†
- Pages: 1052-1055
- First Published: 26 February 2003
A Versatile Catalyst for the Sonogashira Coupling of Aryl Chlorides†
- Pages: 1056-1058
- First Published: 26 February 2003
Hydrogen-Bonded Sugar-Alcohol Trimers as Hexadentate Silicon Chelators in Aqueous Solution†
- Pages: 1058-1062
- First Published: 26 February 2003
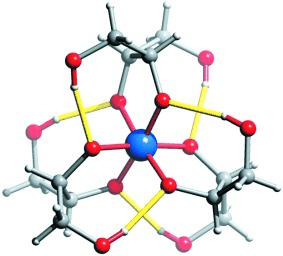
Building bridges: Fumed colloidal silica can be dissolved in alkaline aqueous solutions of the open-chain sugar alcohols mannitol, xylitol, and threitol. These polyols are able to form six-coordinate silicon complexes that are stabilized by intramolecular hydrogen bonds. The prototypical core is shown (yellow bars: the intramolecular H bonds).





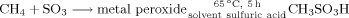
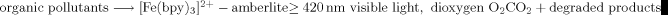
![The Fluoroacyltris(trifluoromethyl)borate Ion, [(CF3)3BC(O)F]−, a Fluoroacylboron Complex](/cms/asset/7c54d8ff-a777-42e1-a565-bcfec080154e/mcontent.jpg)



































