Organic Templates for the Generation of Inorganic Materials†
Kjeld J. C. van Bommel Dr.
Chemotransfiguration Project, Japan Science and Technology Corporation (JST), 2432 Aikawa, Kurume, Fukuoka 839-0861, Japan
Current address:, BiOMaDe Technology Foundation, Nijenborgh 4, 9747 AG Groningen, Netherlands, Both authors contributed equally to this paper.
Search for more papers by this authorArianna Friggeri Dr.
Chemotransfiguration Project, Japan Science and Technology Corporation (JST), 2432 Aikawa, Kurume, Fukuoka 839-0861, Japan
Current address:, BiOMaDe Technology Foundation, Nijenborgh 4, 9747 AG Groningen, Netherlands, Both authors contributed equally to this paper.
Search for more papers by this authorSeiji Shinkai Prof.
Chemotransfiguration Project, Japan Science and Technology Corporation (JST), 2432 Aikawa, Kurume, Fukuoka 839-0861, Japan
Search for more papers by this authorKjeld J. C. van Bommel Dr.
Chemotransfiguration Project, Japan Science and Technology Corporation (JST), 2432 Aikawa, Kurume, Fukuoka 839-0861, Japan
Current address:, BiOMaDe Technology Foundation, Nijenborgh 4, 9747 AG Groningen, Netherlands, Both authors contributed equally to this paper.
Search for more papers by this authorArianna Friggeri Dr.
Chemotransfiguration Project, Japan Science and Technology Corporation (JST), 2432 Aikawa, Kurume, Fukuoka 839-0861, Japan
Current address:, BiOMaDe Technology Foundation, Nijenborgh 4, 9747 AG Groningen, Netherlands, Both authors contributed equally to this paper.
Search for more papers by this authorSeiji Shinkai Prof.
Chemotransfiguration Project, Japan Science and Technology Corporation (JST), 2432 Aikawa, Kurume, Fukuoka 839-0861, Japan
Search for more papers by this authorThe pasta images in the frontispieceare reproduced with permission from “The Cooks Thesaurus” (http://www.foodsubs.com).
Graphical Abstract
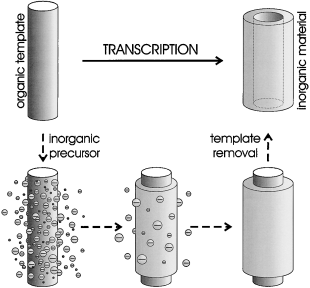
Shapely structured inorganic materials of diverse morphologies and compositions are nowadays obtainable through the transcription of organic templates (see picture). This review gives an overview of the various kinds of templates, precursors, and methods that have been employed for this purpose, as well as the diversity of inorganic materials that have been obtained.
Abstract
Mankind's fascination with shapes and patterns, many examples of which come from nature, has greatly influenced areas such as art and architecture. Science too has long since been interested in the origin of shapes and structures found in nature. Whereas organic chemistry in general, and supramolecular chemistry especially, has been very successful in creating large superstructures of often stunning morphology, inorganic chemistry has lagged behind. Over the last decade, however, researchers in various fields of chemistry have been studying novel methods through which the shape of inorganic materials can be controlled at the micro- or even nanoscopic level. A method that has proven very successful is the formation of inorganic structures under the influence of (bio)organic templates, which has resulted in the generation of a large variety of structured inorganic structures that are currently unattainable through any other method.
References
- 1For recent reviews, see
- 1aN. K. Raman, M. T. Anderson, C. J. Brinker, Chem. Mater. 1996, 8, 1682–1701;
- 1bS. Mann, S. L. Burkett, S. A. Davis, C. E. Fowler, N. H. Mendelson, S. D. Sims, D. Walsh, N. T. Whilton, Chem. Mater. 1997, 9, 2300–2310;
- 1cL. A. Estroff, A. D. Hamilton, Chem. Mater. 2001, 13, 3227–3235;
- 1dS. A. Davis, M. Breulmann, K. H. Rhodes, B. Zhang, S. Mann, Chem. Mater. 2001, 13, 3218–3226;
- 1eR. A. Caruso, M. Antonietti, Chem. Mater. 2001, 13, 3272–3282.
- 2For recent reviews, see
- 2aJ. H. van Esch, B. L. Feringa, Angew. Chem. 2000, 112, 2351–2354;
Angew. Chem. Int. Ed. 2000, 39, 2263–2265;
10.1002/1521-3773(20000703)39:13<2263::AID-ANIE2263>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 2bD. J. Abdallah, R. G. Weiss, Adv. Mater. 2000, 12, 1237–1247;
- 2cG. F. Swiegers, T. J. Malefetse, Chem. Rev. 2000, 100, 3483–3538;
- 2dD. T. Bong, T. D. Clark, J. R. Granja, M. R. Ghadiri, Angew. Chem. 2001, 113, 1016–1041;
10.1002/1521-3757(20010316)113:6<1016::AID-ANGE10160>3.0.CO;2-8 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 988–1011;10.1002/1521-3773(20010316)40:6<988::AID-ANIE9880>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 2eL. J. Prins, D. N. Reinhoudt, P. Timmerman, Angew. Chem. 2001, 113, 2446–2492;
10.1002/1521-3757(20010702)113:13<2446::AID-ANGE2446>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2382–2426;10.1002/1521-3773(20010702)40:13<2382::AID-ANIE2382>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 2fB. J. Holliday, C. A. Mirkin, Angew. Chem. 2001, 113, 2076–2097;
10.1002/1521-3757(20010601)113:11<2076::AID-ANGE2076>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2022–2043.10.1002/1521-3773(20010601)40:11<2022::AID-ANIE2022>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 3P. A. Buining, B. M. Humbel, A. P. Philipse, A. J. Verkleij, Langmuir 1997, 13, 3921–3926.
- 4M. Giersig, T. Ung, L. M. Liz-Márzan, P. Mulvaney, Adv. Mater. 1997, 9, 570–575.
- 5M. Kishida, T. Tago, T. Hatsuta, K. Wakabayashi, Chem. Lett. 2000, 1108–1109.
- 6S. Santra, R. Tapec, N. Theodoropoulou, J. Dobson, A. Hebart, W. Tan, Langmuir 2001, 17, 2900–2906.
- 7For a review on the templated formation of porous silica, see ref. [1 a].
- 8N. Kawahashi, E. Matijević, J. Colloid Interface Sci. 1991, 143, 103–110.
- 9K. W. Gallis, C. C. Landry, Adv. Mater. 2001, 13, 23–26.
- 10Y. Lu, Y. Yin, Y. Xia, Adv. Mater. 2001, 13, 271–274.
- 11C. Sanchez, G. J. de A. A. Soler-Illia, F. Ribot, T. Lalot, C. R. Mayer, V. Cabuil, Chem. Mater. 2001, 13, 3061–3083, and references therein.
- 12P. Innocenzi, G. Brusatin, Chem. Mater. 2001, 13, 3126–3139, and references therein.
- 13
- 13aF. Caruso, Adv. Mater. 2001, 13, 11–22;
- 13bF. Caruso, Chem. Eur. J. 2000, 6, 413–419.
10.1002/(SICI)1521-3765(20000204)6:3<413::AID-CHEM413>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 14M. L. Breen, A. D. Dinsmore, R. H. Pink, S. B. Qadri, B. R. Ratna, Langmuir 2001, 17, 903–907.
- 15A. Imhof, Langmuir 2001, 17, 3579–3585, and references therein.
- 16H. Shiho, N. Kawahashi, Colloid Polym. Sci. 2000, 278, 270–274.
- 17B. zu Putlitz, K. Landfester, H. Fischer, M. Antonietti, Adv. Mater. 2001, 13, 500–503.
- 18H. Bamnolker, B. Nitzan, S. Gura, S. Margel, J. Mater. Sci. Lett. 1997, 16, 1412–1415.
- 19F. Caruso, M. Spasova, A. Susha, M. Giersig, R. A. Caruso, Chem. Mater. 2001, 13, 109–116.
- 20F. Caruso, R. A. Caruso, H. Möhwald, Science 1998, 282, 1111–1114, and references therein.
- 21
- 21aF. Caruso, R. A. Caruso, H. Möhwald, Chem. Mater. 1999, 11, 3309–3314;
- 21bF. Caruso, H. Lichtenfeld, M. Giersig, H. Möhwald, J. Am. Chem. Soc. 1998, 120, 8523–8524;
- 21cF. Caruso, H. Möhwald, Langmuir 1999, 15, 8276–8281.
- 22X. D. Wang, W. L. Yang, Y. Tang, Y. J. Wang, S. K. Fu, Z. Gao, Chem. Commun. 2000, 2161–2162.
- 23R. A. Caruso, A. Susha, F. Caruso, Chem. Mater. 2001, 13, 400–409.
- 24L. Tosheva, J. Sterte, Chem. Commun. 2001, 1112–1113.
- 25F. Iskandar, Mikrajuddin, K. Okuyama, Nano Lett. 2001, 1, 231–234.
- 26M. Bognitzki, H. Hou, M. Ishaque, T. Frese, M. Hellwig, C. Schwarte, A. Schaper, J. H. Wendorff, A. Greiner, Adv. Mater. 2000, 12, 637–640.
- 27K. J. C. van Bommel, J. H. Jung, S. Shinkai, Adv. Mater. 2001, 13, 1472–1476, and references therein.
- 28T. Coradin, E. Mercey, L. Linsnard, J. Livage, Chem. Commun. 2001, 2496–2497.
- 29J. N. Cha, G. D. Stucky, D. E. Morse, T. J. Deming, Nature 2000, 403, 289–292.
- 30K. Shimizu, J. Cha, G. D. Stucky, D. E. Morse, Proc. Natl. Acad. Sci. USA 1998, 95, 6234–6238.
- 31Y. Zhou, K. Shimizu, J. N. Cha, G. D. Stucky, D. E. Morse, Angew. Chem. 1999, 111, 826–828;
Angew. Chem. Int. Ed. 1999, 38, 780–782 and references therein.
10.1002/(SICI)1521-3773(19990315)38:6<779::AID-ANIE779>3.0.CO;2-# CAS Web of Science® Google Scholar
- 32C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli, J. S. Beck, Nature 1992, 359, 710–712.
- 33O. R. Anderson in Biomineralization in Lower Plants and Animals, Vol. 30 (Eds.: B. S. C. Leadbeater, R. Riding), Oxford University Press, Oxford, 1986, pp. 375–391.
- 34S. Mann, G. A. Ozin, Nature 1996, 382, 313–318.
- 35D. Walsh, S. Mann, Nature 1995, 377, 320–323.
- 36D. Walsh, B. Lebeau, S. Mann, Adv. Mater. 1999, 11, 324–328.
- 37D. Walsh, J. D. Hopwood, S. Mann, Science 1994, 264, 1576–1578.
- 38P. S. Singh, K. Kosuge, Chem. Lett. 1998, 101–102.
- 39S. S. Kim, W. Zhang, T. J. Pinnavaia, Science 1998, 282, 1302–1305.
- 40P. T. Tanev, Y. Liang, T. Pinnavaia, J. Am. Chem. Soc. 1997, 119, 8616–8624.
- 41Y. Lu, H. Fan, A. Stump, T. L. Ward, T. Rieker, C. J. Brinker, Nature 1999, 398, 223–226.
- 42
- 42aD. H. W. Hubert, M. Jung, P. M. Frederik, P. H. H. Bomans, J. Meuldijk, A. L. German, Adv. Mater. 2000, 12, 1286–1290;
- 42bD. H. W. Hubert, M. Jung, A. L. German, Adv. Mater. 2000, 12, 1291–1294.
- 43H.-P. Lin, Y.-R. Cheng, C.-Y. Mou, Chem. Mater. 1998, 10, 3772–3776.
- 44S. Oliver, A. Kuperman, N. Coombs, A. Lough, G. A. Ozin, Nature 1995, 378, 47–50, and references therein.
- 45K. Katagiri, K. Ariga, J.-i. Kikuchi, Chem. Lett. 1999, 661–662.
- 46S. Baral, P. Schoen, Chem. Mater. 1993, 5, 145–147.
- 47D. D. Archibald, S. Mann, Nature 1993, 364, 430–433.
- 48
- 48aM. Adachi, T. Harada, M. Harada, Langmuir 1999, 15, 7097–7100;
- 48bM. Adachi, T. Harada, M. Harada, Langmuir 2000, 16, 2376–2384;
- 48cM. Harada, M. Adachi, Adv. Mater. 2000, 12, 839–841.
- 49M. Niederberger, H.-J. Muhr, F. Krumeich, F. Bieri, D. Günther, R. Nesper, Chem. Mater. 2000, 12, 1995–2000.
- 50H. Matsui, S. Pan, B. Gologan, S. H. Jonas, J. Phys. Chem. B 2000, 104, 9576–9579.
- 51L. Qi, J. Ma, H. Cheng, Z. Zhao, J. Phys. Chem. B 1997, 101, 3460–3463.
- 52P. Terech, R. G. Weiss, Chem. Rev. 1997, 97, 3133–3159.
- 53S. Shinkai, K. Murata, J. Mater. Chem. 1998, 8, 485–495.
- 54O. Gronwald, S. Shinkai, Chem. Eur. J. 2001, 7, 4328–4334.
10.1002/1521-3765(20011015)7:20<4328::AID-CHEM4328>3.0.CO;2-S CAS PubMed Web of Science® Google Scholar
- 55V. Jenning, A. Gysler, M. Schafer-Korting, S. H. Gohla, Eur. J. Pharm. Biopharm. 2000, 49, 211–218.
- 56C. Bergeret-Galley, X. Latouche, Y. G. Illouz, Aesthet. Plast. Surg. 2001, 25, 249–255.
- 57D. Duke, J. M. Grevelink, Dermatol. Surg. 1998, 24, 201–206.
- 58S. H. Hyon, W. I. Cha, Y. Ikada, M. Kita, Y. Ogura, Y. Honda, J. Biomater. Sci. Polym. Ed. 1994, 5, 397–406.
- 59L. Alvord, J. Court, T. Davis, C. F. Morgan, K. Schindhelm, J. Vogt, L. Winterton, Optometry Vision Sci. 1998, 75, 30–36.
- 60W. Kubo, K. Murakoshi, T. Kitamura, Y. Wada, K. Hanabusa, H. Shirai, S. Yanagida, Chem. Lett. 1998, 1241–1242.
- 61K. Hanabusa, K. Hiratsuka, M. Kimura, H. Shirai, Chem. Mater. 1999, 11, 649–655.
- 62Y. Ono, K. Nakashima, M. Sano, Y. Kanekiyo, K. Inoue, J. Hojo, S. Shinkai, Chem. Commun. 1998, 1477–1478.
- 63For a review on the gelation of liquids by cholesterol derivatives, see ref. [53]. The aggregation structure of these gels has been studied by using small angle X-ray scattering: K. Sakurai, Y. Ono, J. H. Jung, S. Okamoto, S. Sakurai, S. Shinkai, J. Chem. Soc. Perkin Trans. 2 2001, 108–112.
- 64K. Murata, Ph. D. Thesis, Graduate School of Engineering, Kyushu University, 1997.
- 65A. Stein, B. J. Melde, R. Schroden, Adv. Mater. 2000, 12, 1403–1419, and references therein.
- 66Typical drying and calcination steps for organogel transcription systems consist of: 3–7 days under vacuum at RT (optional), 2 h at 100–200 °C (depending on the solvent) under N2, 2 h at 500 °C under N2, 4 h at 500 °C under aerobic conditions.
- 67C. J. Brinker, G. W. Scherer, Sol-Gel Science, Academic Press, San Diego, 1990.
10.1016/B978-0-08-057103-4.50013-1 Google Scholar
- 68Y.-C. Lin, R. G. Weiss, Macromolecules 1987, 20, 414–417.
- 69Y. Ono, K. Nakashima, M. Sano, J. Hojo, S. Shinkai, Chem. Lett. 1999, 1119–1120.
- 70Y. Ono, K. Nakashima, M. Sano, J. Hojo, S. Shinkai, J. Mater. Chem. 2001, 11, 2412–2419.
- 71Y. Ono, Y. Kanekiyo, K. Inoue, J. Hojo, S. Shinkai, Chem. Lett. 1999, 23–24.
- 72This experiment was carried out in BuOH, which necessitated the use of benzylamine as a catalyst for TEOS polycondensation. It also acted as the catalyst in all experiments where acetic acid was used as the solvent.
- 73J. H. Jung, Y. Ono, S. Shinkai, J. Chem. Soc. Perkin Trans. 2 1999, 1289–1291.
- 74J. H. Jung, Y. Ono, S. Shinkai, Langmuir 2000, 16, 1643–1649.
- 75J. H. Jung, Y. Ono, S. Shinkai, Chem. Lett. 2000, 636–637.
- 76J. H. Jung, S. Shinkai, J. Chem. Soc. Perkin Trans. 2 2000, 2393–2398.
- 77J. H. Jung, Y. Ono, S. Shinkai, Angew. Chem. 2000, 112, 1931–1933;
10.1002/(SICI)1521-3757(20000515)112:10<1931::AID-ANGE1931>3.0.CO;2-B Web of Science® Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1862–1865.10.1002/(SICI)1521-3773(20000515)39:10<1862::AID-ANIE1862>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 78J. H. Jung, H. Kobayashi, M. Masuda, T. Shimizu, S. Shinkai, J. Am. Chem. Soc. 2001, 123, 8785–8789.
- 79L. E. Echegoyen, J. Hernandez, A. E. Kaifer, G. W. Gokel, L. Echegoyen, J. Chem. Soc. Chem. Commun. 1988, 836–837.
- 80J. H. Jung, Y. Ono, K. Sakurai, M. Sano, S. Shinkai J. Am. Chem. Soc. 2000, 122, 8648–8653.
- 81J. H. Jung, K. Nakashima, S. Shinkai, Nano Lett. 2001, 1, 145–148.
- 82K. Hanabusa, K. Shimura, K. Hirose, M. Kimura, H. Shirai, Chem. Lett. 1996, 885–886.
- 83J. H. Jung, Y. Ono, K. Hanabusa, S. Shinkai, J. Am. Chem. Soc. 2000, 122, 5008–5009.
- 84J. H. Jung, Y. Ono, S. Shinkai, Chem. Eur. J. 2000, 6, 4552–4557.
10.1002/1521-3765(20001215)6:24<4552::AID-CHEM4552>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 85No gelation occurred at noncharged:charged ratios of <15:85; only granular silica was obtained.
- 86S. Kobayashi, K. Hanabusa, N. Hamasaki, M. Kimura, H. Shirai, S. Shinkai, Chem. Mater. 2000, 12, 1523–1525.
- 87J. J. E. Moreau, L. Vellutini, M. Wong Chi Man, C. Bied, J. Am. Chem. Soc. 2001, 123, 1509–1510.
- 88In fact, this system no longer involves templation; it is based on a polymerization through which the superstructure is covalently captured.
- 89J. J. E. Moreau, L. Vellutini, M. Wong Chi Man, C. Bied, J.-L. Bantignies, P. Dieudonné, J.-L. Sauvajol, J. Am. Chem. Soc. 2001, 123, 7957–7958.
- 90U. Beginn, S. Keinath, M. Möller, Macromol. Chem. Phys. 1998, 199, 2379–2384.
10.1002/(SICI)1521-3935(19981101)199:11<2379::AID-MACP2379>3.0.CO;2-2 CAS Web of Science® Google Scholar
- 91K. Yoza, Y. Ono, K. Yoshihara, T. Akao, H. Shinmori, M. Takeuchi, S. Shinkai, D. Reinhoudt, Chem. Commun. 1998, 907–908.
- 92R. J. H. Hafkamp, M. C. Feiters, R. J. M. Nolte, J. Org. Chem. 1999, 64, 412–426.
- 93K. Yoza, Y. Ono, K. Yoshihara, T. Akao, H. Shinmori, M. Takeuchi, S. Shinkai, D. N. Reinhoudt, Chem. Commun. 1998, 907–908.
- 94N. Amanokura, Y. Kanekiyo, S. Shinkai, D. N. Reinhoudt, J. Chem. Soc. Perkin Trans. 2 1999, 1995–2000.
- 95J. H. Jung, M. Amaike, S. Shinkai, Chem. Commun. 2000, 2343–2344.
- 96J. H. Jung, M. Amaike, K. Nakashima, S. Shinkai, J. Chem. Soc. Perkin Trans. 2 2001, 1938–1943.
- 97J. H. Jung, M. Amaike, S. Shinkai, Trans. Mater. Res. Soc. Jap. 2001, 26, 527–530.
- 98N. Amanokura, Y. Kanekiyo, S. Shinkai, D. N. Reinhoudt, J. Chem. Soc. Perkin Trans. 2 1999, 1995–2000.
- 99This was confirmed by TEM analysis of the original organogels, in which the α-glucose derivative formed a frizzled fibrous network with fiber diameters of about 5 to 20 nm. The β-glucose derivative on the other hand formed thick fibers with diameters ranging from 50 to 150 nm.
- 100K. Sakurai, A. Friggeri, O. Gronwald, S. Sakurai, S. Okamoto, K. Inoue, S. Shinkai, unpublished results.
- 101O. Gronwald, S. Shinkai, J. Chem. Soc. Perkin Trans. 2 2001, 1933–1937.
- 102A. Friggeri, O. Gronwald, K. J. C. van Bommel, S. Shinkai, D. N. Reinhoudt, Chem. Commun. 2001, 2434–2435.
- 103So far, transcription of gelators bearing neutral amine groups has only been performed in the case of a primary amine or a combination of a primary and secondary amine (see, for example, refs. [75, 95]).
- 104K. J. C. van Bommel, S. Shinkai, Langmuir 2002, 18, 4544–4548.
- 105In a very recent article Stupp and co-workers describe the innovative preparation of (racemic mixtures of) CdS helices under the influence of an organogel template: E. D. Sone, E. R. Zubarev, S. I. Stupp, Angew. Chem. 2002, 114, 1781–1785;
10.1002/1521-3757(20020517)114:10<1781::AID-ANGE1781>3.0.CO;2-W Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1705–1709.
- 106W. Kratschmer, L. D. Lamb, K. Fostiropoulos, R. D. Huffman, Nature 1990, 347, 354–358.
- 107S. Iijima, Nature 1991, 354, 56–58.
- 108For an overview on nanotubes, see C. N. R. Rao, B. C. Satishkumar, A. Govindaraj, M. Nath, ChemPhysChem 2001, 2, 78–105, and references therein.
- 109G. Che, B. B. Lakshmi, C. R. Martin, E. R. Fisher, Langmuir 1999, 15, 750–758.
- 110B. Xue, P. Chen, Q. Hong, J. Lin, K. L. Tan, J. Mater. Chem. 2001, 11, 2378–2381.
- 111M. R. Pederson, J. Q. Broughton, Phys. Rev. Lett. 1992, 69, 2689–2692.
- 112P. M. Ajayan, S. Iijima, Nature 1993, 361, 333–334.
- 113P. M. Ajayan, O. Stephan, P. Redlich, C. Colliex, Nature 1995, 375, 564–567.
- 114S. C. Tsang, Y. K. Chen, P. J. F. Harris, M. L. H. Green, Nature 1994, 372, 159–162.
- 115S. Seraphin, D. Zhou, J. Jiao, J. C. Withers, R. Loutfy, Nature 1993, 362, 503.
- 116C. N. R. Rao, R. Sen, B. C. Satishkumar, A. Govindaraj, Chem. Commun. 1998, 1525–1526.
- 117R. Andrews, D. Jacques, A. M. Rao, F. Derbyshire, D. Qian, X. Fan, E. C. Dickey, J. Chen, Chem. Phys. Lett. 1999, 303, 467–474.
- 118C. N. R. Rao, A. Govindaraj, R. Sen, B. C. Satishkumar, Mater. Res. Innovations 1998, 2, 128–141.
- 119Frequently an aluminum oxide layer formed on an aluminum plate is employed.[120]
- 120J. C. Hulteen, C. R. Martin, J. Mater. Chem. 1997, 7, 1075–1087.
- 121T. Kyotani, L. Tsai, A. Tomita, Chem. Commun. 1997, 701–702.
- 122B. K. Pradhan, T. Kyotani, A. Tomita, Chem. Commun. 1999, 1317–1318.
- 123K. Matsui, B. K. Pradhan, T. Kyotani, A. Tomita, J. Phys. Chem. B 2001, 105, 5682–5688.
- 124B. C. Satishkumar, A. Govindaraj, E. M. Vogl, L. Basumallick, C. N. R. Rao, J. Mater. Res. 1997, 12, 604–606.
- 125The acid treatment results in the formation of acidic sites on the surface of the CNTs.[114]
- 126C. N. R. Rao, B. C. Satishkumar, A. Govindaraj, Chem. Commun. 1997, 1581–1582.
- 127B. C. Satishkumar, A. Govindaraj, M. Nath, C. N. R. Rao, J. Mater. Chem. 2000, 10, 2115–2119.
- 128This deposition method is very similar to the one employed by Caruso.[13]
- 129T. Seeger, P. Redlich, N. Grobert, M. Terrones, D. R. M. Walton, H. W. Kroto, M. Rühle, Chem. Phys. Lett. 2001, 339, 41–46.
- 130Y. Q. Zhu, W. K. Hsu, H. W. Kroto, D. R. M. Walton, Chem. Commun. 2001, 2184–2185.
- 131P. Chen, X. Wu, J. Lin, K. L. Tan, J. Phys. Chem. B 1999, 103, 4559–4561.
- 132S. Fullam, D. Cottell, H. Rensmo, D. Fitzmaurice, Adv. Mater. 2000, 12, 1430–1432.
- 133H. Dai, E. W. Wong, Y. Z. Lu, S. Fan, C. M. Lieber, Nature 1995, 375, 769–772.
- 134E. W. Wong, B. W. Maynor, L. D. Burns, C. M. Lieber, Chem. Mater. 1996, 8, 2041–2046.
- 135W. Han, S. Fan, Q. Li, Y. Hu, Science 1997, 277, 1287–1289.
- 136W. Han, S. Fan, Q. Li, B. Gu, X. Zhang, D. Yu, Appl. Phys. Lett. 1997, 71, 2271–2273.
- 137Y. Zhang, J. Zhu, Q. Zhang, Y. Yan, N. Wang, X. Zhang, Chem. Phys. Lett. 2000, 317, 504–509.
- 138H. Nakamura, Y. Matsui, J. Am. Chem. Soc. 1995, 117, 2651–2652.
- 139F. Miyaji, S. A. Davis, J. P. H. Charmant, S. Mann, Chem. Mater. 1999, 11, 3021–3024.
- 140F. Miyaji, Y. Tatematsu, Y. Suyama, J. Ceram. Soc. Jpn. 2001, 109, 924–928.
- 141C. Hippe, M. Wark, E. Lork, G. Schulz-Ekloff, Microporous Mesoporous Mater. 1999, 31, 235–239.
- 142Y. Ono, Y. Kanekiyo, K. Inoue, J. Hojo, M. Nango, S. Shinkai, Chem. Lett. 1999, 475–476.
- 143E. Braun, Y. Eichen, U. Sivan, G. Ben-Yoseph, Nature 1998, 391, 775–778.
- 144G. Stubbs, Semin. Virol. 1990, 1, 450.
- 145K. Namba, G. Stubbs, Acta Crystallogr. Sect. A 1985, 41, 252–262.
- 146W. Shenton, T. Douglas, M. Young, G. Stubbs, S. Mann, Adv. Mater. 1999, 11, 253–256.
- 147C. E. Fowler, W. Shenton, G. Stubbs, S. Mann, Adv. Mater. 2001, 13, 1266–1269.
- 148S. A. Davis, S. L. Burkett, N. H. Mendelson, S. Mann, Nature 1997, 385, 420–423.
- 149S. Chia, J. Urano, F. Tamanoi, B. Dunn, J. I. Zink, J. Am. Chem. Soc. 2000, 122, 6488–6489.
- 150J. R. Premkumar, O. Lev, R. Rosen, S. Belkin, Adv. Mater. 2001, 13, 1773–1775.
- 151W. Ogasawara, W. Shenton, S. A. Davis, S. Mann, Chem. Mater. 2000, 12, 2835–2837.
- 152Y. Shin, J. Liu, J. H. Chang, Z. Nie, G. J. Exarhos, Adv. Mater. 2001, 13, 728–732.
- 153
- 153aP. Greil, T. Lifka, A. Kaindl, J. Eur. Ceram. Soc. 1998, 18, 1961–1973;
- 153bP. Greil, T. Lifka, A. Kaindl, J. Eur. Ceram. Soc. 1998, 18, 1975–1983;
- 153cP. Greil, J. Eur. Ceram. Soc. 2001, 21, 105–118.