Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture
- Page: 1213
- First Published: 01 May 2025
One-pot sequential reaction combining bimetallic Cu/Ir-catalyzed asymmetric allylation of ketimine esters and (E)-4-indolyl allyl carbonates with acid-mediated Pictet-Spengler cyclization was successfully developed. This protocol enables stereodivergent access to four stereoisomers of indole-fused 9-azabicyclo[4.2.1]nonanes, containing an eight-membered ring system bearing one tertiary and two quaternary stereocenters. It features a remarkable step economy, broad substrate compatibility, and excellent stereoselective control. More details are discussed in the article by Wang et al. on pages 1223—1229.
Inside Cover Picture
Inside Cover Picture
- Page: 1214
- First Published: 01 May 2025

Accurate prediction of chemical reaction performance is crucial for automated chemical synthesis. In this study, a novel multi-modal chemical reaction prediction model is proposed, using graph and textual information, eliminating the need for computationally intensive DFT parameters with capability to handling reactions involving a fluctuating number of molecules. This model outperforms at least 7 generalized methods on 4 datasets, while maintaining chemical interpretability towards atomic and feature importance. Further details are comprehensively discussed in the article by Li et al. on pages 1230—1238.
Contents
Breaking Report
Dual Cu/Ir Catalyzed Asymmetric Allylation and Pictet-Spengler Cyclization: Stereodivergent Access to Chiral Indole Fused 9-Azabicyclo[4.2.1]nonanes
- Pages: 1223-1229
- First Published: 24 February 2025
![Dual Cu/Ir Catalyzed Asymmetric Allylation and Pictet-Spengler Cyclization: Stereodivergent Access to Chiral Indole Fused 9-Azabicyclo[4.2.1]nonanes†](/cms/asset/0b36719a-31eb-4b30-a35a-4820f33925b5/cjoc202500016-toc-0001-m.jpg)
Bimetallic Cu/Ir catalyzed asymmetric allylation of ketimine esters and (E)-4-indolyl allyl carbonates followed by acid-promoted Pictet-Spengler cyclization sequences was developed, enabling stereodivergent synthesis of chiral indole fused 9-azabicyclo[4.2.1]nonanes containing an eight-membered ring with one tertiary and two quaternary stereogenic centers.
Concise Report
Multi-modal Homogeneous Chemical Reaction Performance Prediction with Graph and Chemical Language Information
- Pages: 1230-1238
- First Published: 20 February 2025
Pd(II)-Catalyzed Selective [4+2] Benzannulations of Pyridones with Alkenes: Diversity-Oriented Synthesis of a Novel Fluorescent Quinolinone
- Pages: 1239-1245
- First Published: 25 February 2025
![Pd(II)-Catalyzed Selective [4+2] Benzannulations of Pyridones with Alkenes: Diversity-Oriented Synthesis of a Novel Fluorescent Quinolinone](/cms/asset/c66eca04-9109-4e4f-8c70-4ad2c773014f/cjoc202500045-toc-0001-m.jpg)
A Pd(II)-catalyzed regioselective [4+2] benzannulation of 2-pyridones with electrondeficient alkenes was presented for diversity-oriented synthesis of quinolinone derivatives. The potential reaction mechanism involved a series of sequential C—H activation reactions or 6π electrocyclization and entailed dehydrogenative aromatization for the transformation from 2-pyridone into quinolinone.
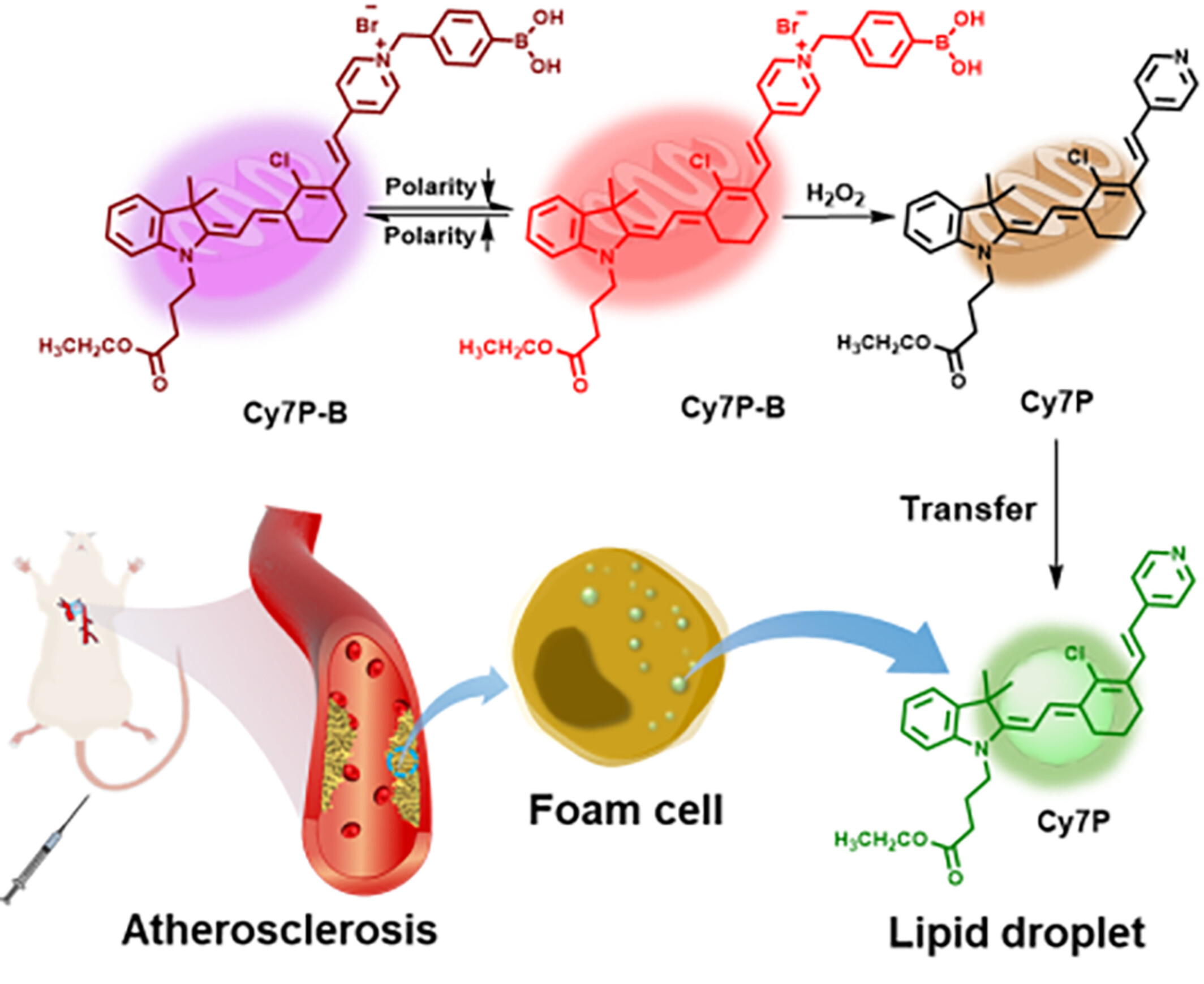
Polarity Sensitive and H2O2/Lipid Droplets Sequence-Activated Asymmetric Cyanine Probe Achieves Multi-marker Imaging of Atherosclerosis
- Pages: 1246-1254
- First Published: 17 March 2025
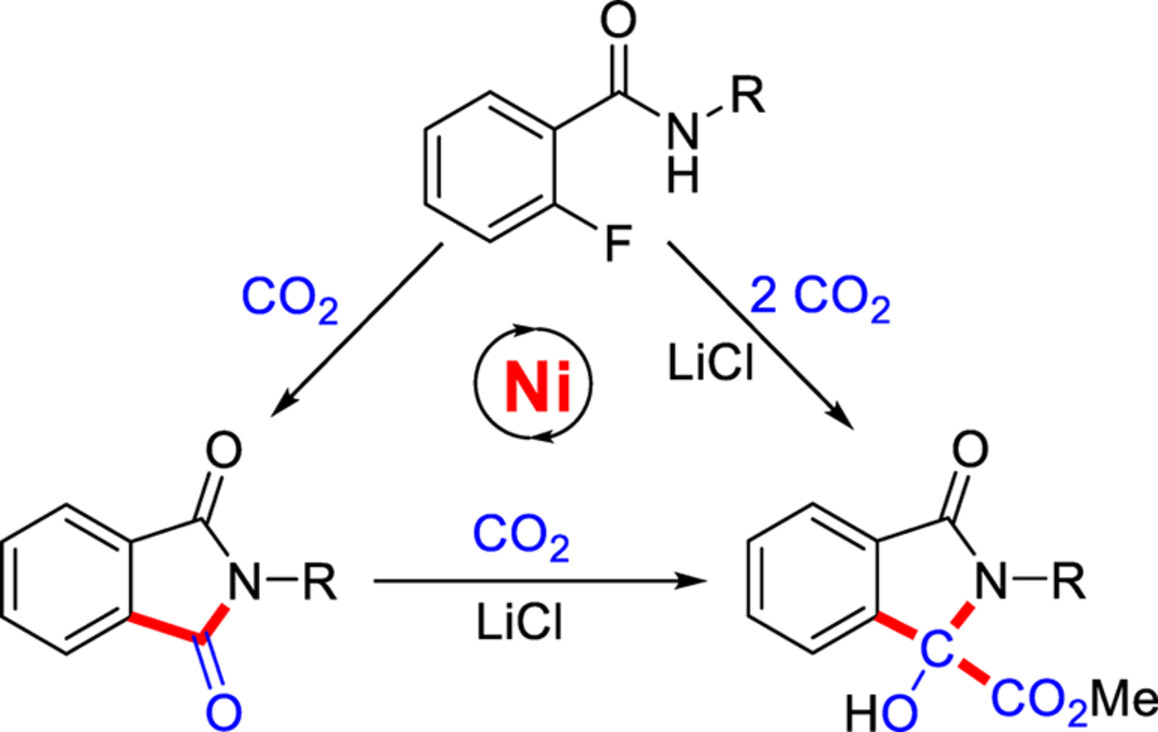
Nickel-Catalyzed LiCl-Controlled Switchable Carboxylation of Aryl C—F Bonds with One or Two Molecules of CO2
- Pages: 1255-1262
- First Published: 07 March 2025
Enantioselective Synthesis of Planar/Multiple Chiral [n]Cyclophanes through Asymmetric Allylation
- Pages: 1263-1270
- First Published: 07 March 2025
![Enantioselective Synthesis of Planar/Multiple Chiral [n]Cyclophanes through Asymmetric Allylation](/cms/asset/105b7ae3-5521-430f-b409-c0d6d5f4e809/cjoc202500010-toc-0001-m.jpg)
The Bi(OAc)3/CPA catalyzed asymmetric allylation reactions of racemic aldehyde [n]cyclophanes via KR have been disclosed, in which planar-chiral [n]cyclophanes with carbon-centered chirality were synthesized with stereoselectivities. The versatility in substrate scope and potential for further transformations substantiate the significance of this methodology. Furthermore, theoretical calculations elucidate the origin of enantioselectivity.
Nickel-Catalyzed Asymmetric Reductive 1,4- and 1,5-Dicarbofunctionalization
- Pages: 1271-1278
- First Published: 08 March 2025
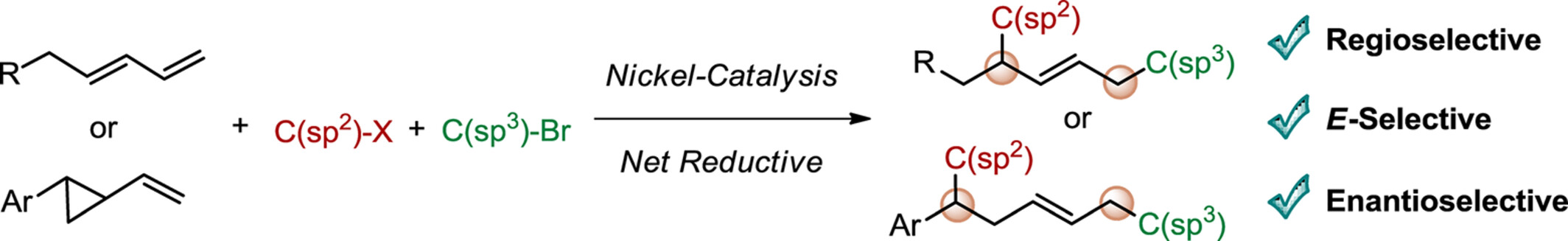
Nickel-catalyzed asymmetric reductive 1,4-dicarbofunctionalization of 1,3-dienes and 1,5-dicarbofunctionalization of vinylcyclopropanes have been disclosed, providing a variety of chiral olefins bearing an allylic or a homoallylic stereogenic center in high regio-, diastereo-, and enantioselectivities.
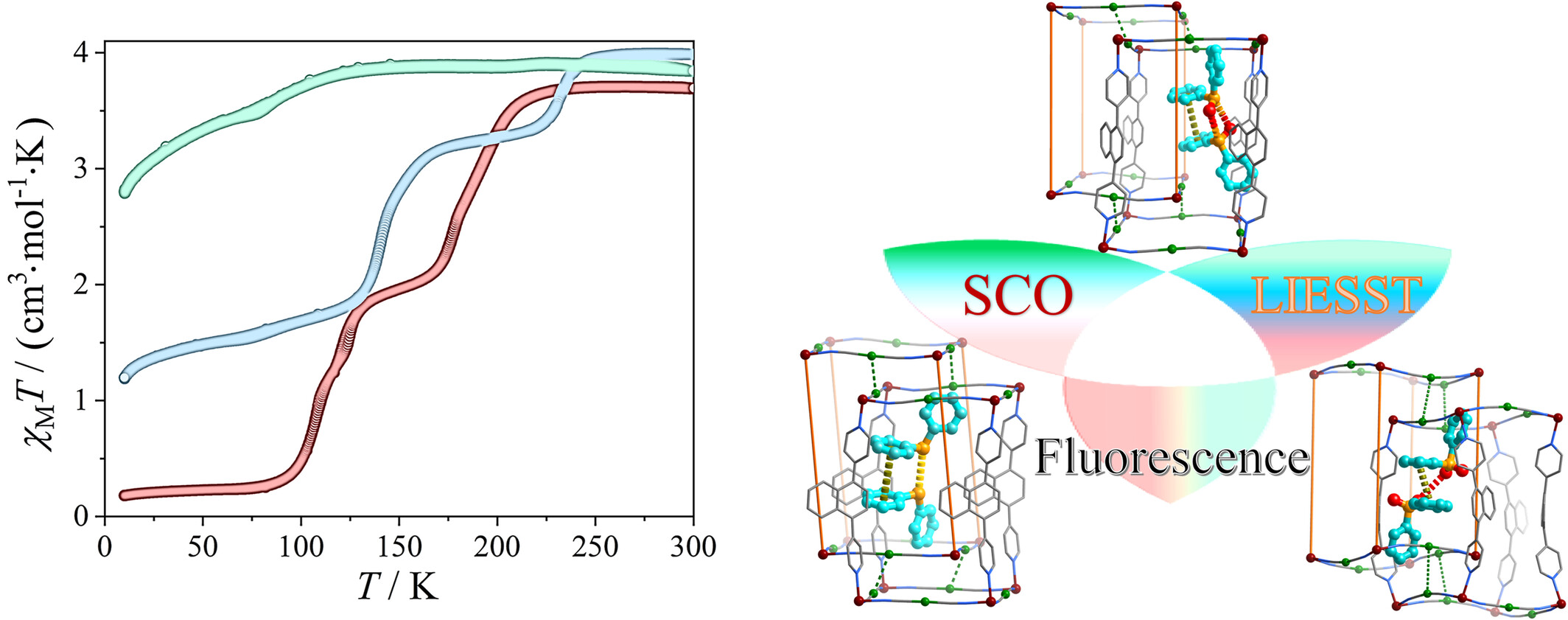
Tuning Spin Crossover Properties in Hofmann-Type Framework by Guest-Adaptive Deformation
- Pages: 1279-1286
- First Published: 08 March 2025
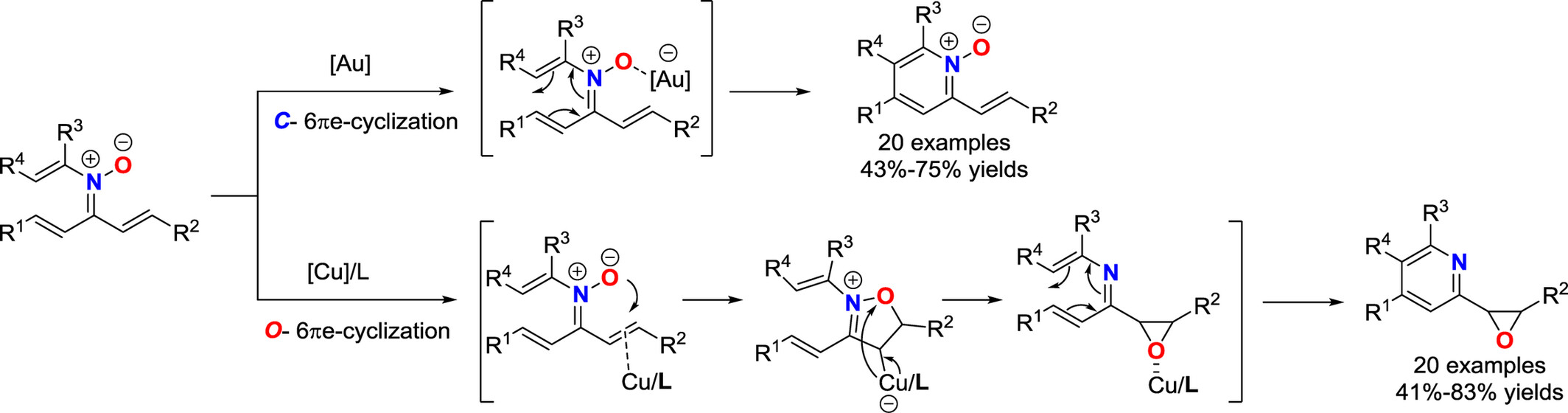
Selective 6π-Electrocyclization of N-Vinyl-α,β-Unsaturated Nitrones to Prepare Polysubstituted Pyridine Derivatives
- Pages: 1287-1292
- First Published: 07 March 2025
Development of Silyl-Protected Phosphoramidite Building Blocks for Short ssDNA Synthesis
- Pages: 1293-1298
- First Published: 08 March 2025
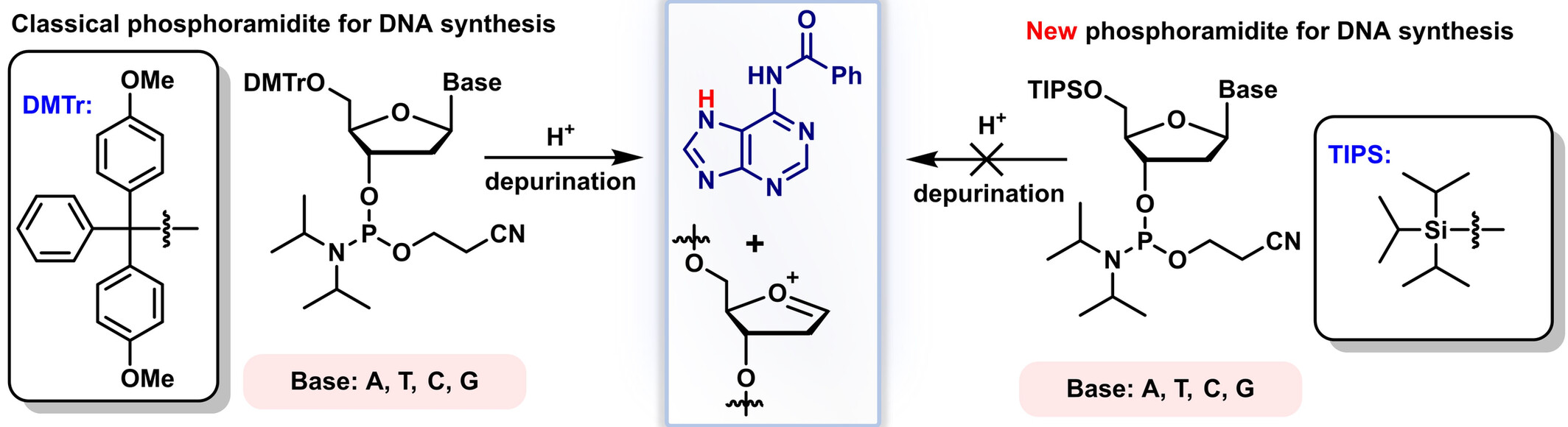
We developed a novel method for synthesizing ssDNA using fluoride-sensitive, triisopropylsilyl (TIPS)-protected phosphoramidite building blocks, achieving over 99% deprotection efficiency under mild, non-acidic conditions. Solid-phase synthesis of short ssDNA sequences yielded over 99% efficiency per cycle, demonstrating high specificity and efficiency.
Neutral Chalcogen Bonding Enabled Photoinduced Cross-Electrophile C—S/Se Coupling of Aryl Iodides via SRN1 Process
- Pages: 1299-1305
- First Published: 07 March 2025
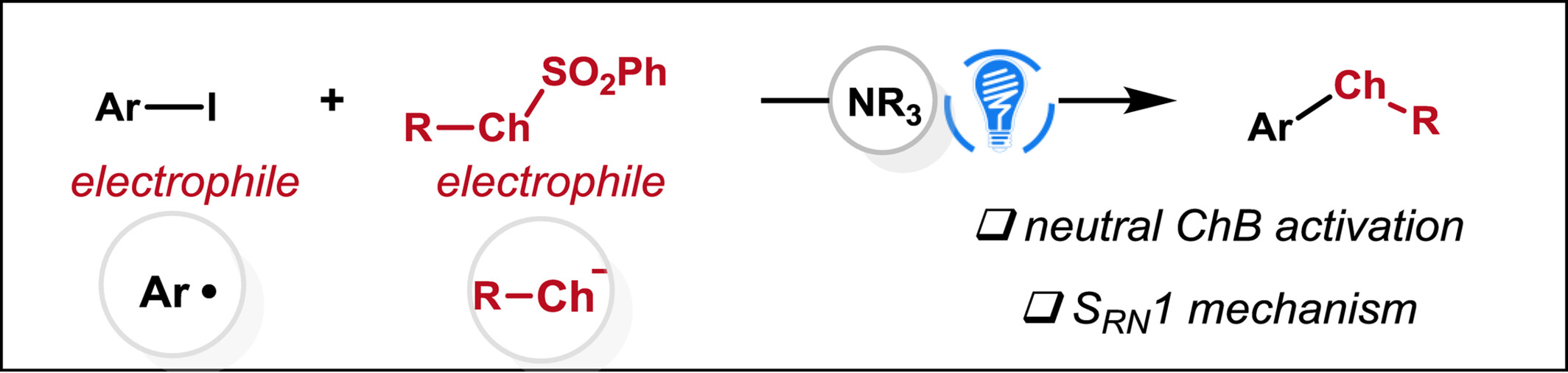
We report a neutral chalcogen bonding-enabled cross-electrophile C-Ch coupling reaction between aryl iodides and selenosulfonates under visible light irradiation. Mechanistic analysis shows that the process involves the photogeneration of R-Se anions and α-aminoalkyl radicals, which facilitate the activation of aryl iodides via halogen atom transfer, ultimately leading to the formation of aryl radicals.
Comprehensive Report
Improving Pure Organic Room-Temperature Phosphorescence by Substituent Effect of Thianthrene
- Pages: 1306-1314
- First Published: 17 March 2025
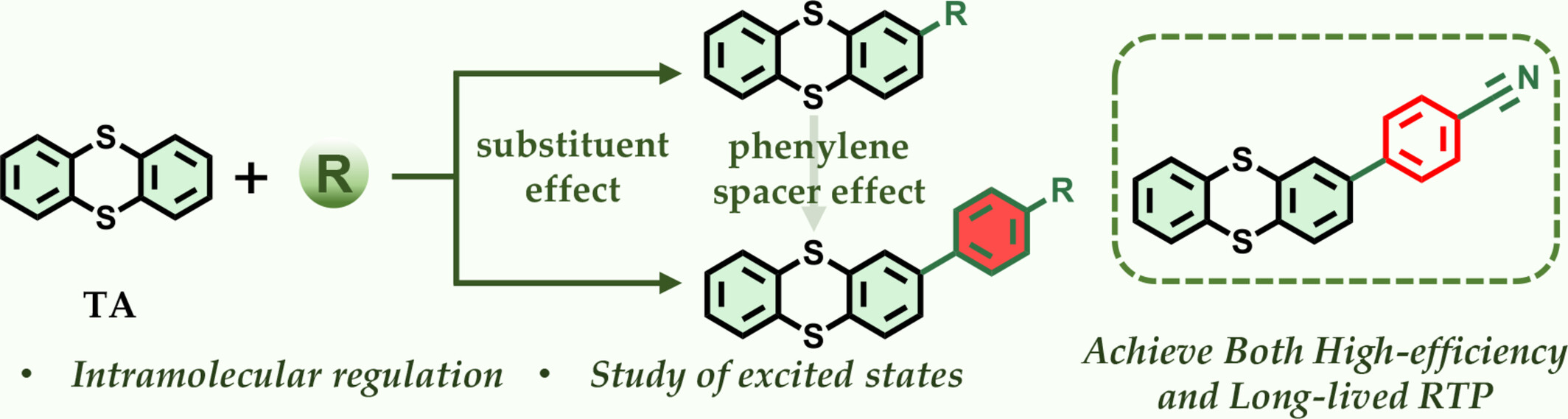
A systematic investigation of substituent effects on the RTP properties of TA revealed that substitution at the 2-position leads to a concurrent enhancement of RTP efficiency and emission lifetime. These optimized RTP materials hold promise for applications in information storage and time-dependent decryption.
Pyrazino[2,3-f][1,10]phenanthroline Derivatives for Oxygen-Tolerant Dual Photoredox/Copper Catalyzed Atom Transfer Radical Polymerization with Ultra-low Catalyst Dosage
- Pages: 1315-1324
- First Published: 20 March 2025
Inside Back Cover
Inside Back Cover
- Page: 1327
- First Published: 01 May 2025

Quinolinone is a widely found partially aromatic heterocycle that is present in numerous natural products and pharmaceutical compounds. This work presents an intermolecular palladium-catalyzed regioselective [4+2] benzannulation reaction capable of converting 2-pyridones into quinolinones using electron-deficient alkenes as two-carbon units. More details are discussed in the article by Xu et al. on pages 1239—1245.
Back Cover
Back Cover
- Page: 1328
- First Published: 01 May 2025

The neutral chalcogen bonding interaction established between chalcogen electrophiles and alkyl amines facilitates the photogeneration of R-Se⁻ anions and α-aminoalkyl radicals via single electron transfer processes. The α-aminoalkyl radicals then abstract halogen atoms, leading to the formation of aryl radicals. Finally, a radical nucleophilic substitution drives the cross-electrophile C-Se coupling reaction. More details are discussed in the article by Chen et al. on pages 1299—1305.






![Pyrazino[2,3-f][1,10]phenanthroline Derivatives for Oxygen-Tolerant Dual Photoredox/Copper Catalyzed Atom Transfer Radical Polymerization with Ultra-low Catalyst Dosage](/cms/asset/3a6b38d1-7ea2-46e1-af57-c49c0b941e18/cjoc70019-toc-0001-m.jpg)




